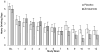Placebo-controlled trial of zonisamide for the treatment of alcohol dependence
- PMID: 20473070
- PMCID: PMC3157084
- DOI: 10.1097/JCP.0b013e3181db38bb
Placebo-controlled trial of zonisamide for the treatment of alcohol dependence
Abstract
Introduction: Zonisamide is an anticonvulsant medication with GABAergic, glutamatergic, and monoaminergic effects. Zonisamide has also been shown to reduce alcohol intake in rodents and in risky drinkers in the context of a laboratory study. This pilot clinical trial evaluated the safety, tolerability, and efficacy of zonisamide for the treatment of alcohol dependence.
Methods: Forty alcohol-dependent subjects (23 men) were randomly assigned to receive treatment with either placebo or zonisamide in a 12-week double-blind trial. Zonisamide was initiated at a dosage of 100 mg/d, which was increased by 100 mg/d every 2 weeks for 8 weeks to a maximum dosage of 500 mg/d. The medication was continued for 4 weeks at the target dosage and then tapered and discontinued. The primary outcomes were drinks per week, heavy drinking days per week, and abstinent days per week, which were measured using the Timeline Follow-Back method.
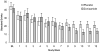
Results: There was a significant medication by treatment week interaction effect favoring the zonisamide group for heavy drinking days (HDD; P = 0.012), drinks per week (P = 0.004), and alcohol urge scores (P = 0.006). There was not a significant effect on the number or rate of increase in abstinent days. There were no serious adverse events reported and zonisamide treatment was well tolerated.
Conclusion: The findings provide preliminary support for the use of zonisamide to treat alcohol dependence. Efforts to replicate and extend these findings are warranted.
Figures
References
-
- Ait-Daoud N, Malcolm RJ, Jr, Johnson BA. An overview of medications for the treatment of alcohol withdrawal and alcohol dependence with an emphasis on the use of older and newer anticonvulsants. Addict Behav. 2006;31(9):1628–49. - PubMed
-
- Johnson BA, et al. Oral topiramate for treatment of alcohol dependence: a randomised controlled trial. Lancet. 2003;361(9370):1677–85. - PubMed
-
- Johnson BA, et al. Topiramate for treating alcohol dependence: a randomized controlled trial. Jama. 2007;298(14):1641–51. - PubMed
-
- White HS. Mechanism of action of newer anticonvulsants. J Clin Psychiatry. 2003;64(Suppl 8):5–8. - PubMed
-
- Perucca E. Clinical pharmacology and therapeutic use of the new antiepileptic drugs. Fundam Clin Pharmacol. 2001;15(6):405–17. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical