Biomarker discovery and development in pediatric critical care medicine
- PMID: 20473243
- PMCID: PMC2924462
- DOI: 10.1097/PCC.0b013e3181e28876
Biomarker discovery and development in pediatric critical care medicine
Abstract
Objectives: To frame the general process of biomarker discovery and development; and to describe a proposal for the development of a multibiomarker-based risk model for pediatric septic shock.
Data source: Narrative literature review and author-generated data.
Data selection: Biomarkers can be grouped into four broad classes, based on the intended function: diagnostic, monitoring, surrogate, and stratification.
Data extraction and synthesis: Biomarker discovery and development requires a rigorous process, which is frequently not well followed in the critical care medicine literature. Very few biomarkers have successfully transitioned from the candidate stage to the true biomarker stage. There is great interest in developing diagnostic and stratification biomarkers for sepsis. Procalcitonin is currently the most promising diagnostic biomarker for sepsis. Recent evidence suggested that interleukin-8 can be used to stratify children with septic shock having a high likelihood of survival with standard care. Currently, there is a multi-institutional effort to develop a multibiomarker-based sepsis risk model intended to predict outcome and illness severity for individual children with septic shock.
Conclusions: Biomarker discovery and development are an important portion of the pediatric critical care medicine translational research agenda. This effort will require collaboration across multiple institutions and investigators. Rigorous conduct of biomarker-focused research holds the promise of transforming our ability to care for individual patients and our ability to conduct clinical trials in a more effective manner.
Figures

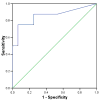
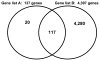
Comment in
-
Biomarkers for sepsis: PERSEVERE to the bitter end.Pediatr Crit Care Med. 2011 Mar;12(2):225-6. doi: 10.1097/PCC.0b013e3181f4d5e5. Pediatr Crit Care Med. 2011. PMID: 21646948 No abstract available.
References
-
- Biomarkers and surrogate endpoints: preferred definitions and conceptual framework. Clin Pharmacol Ther. 2001;69(3):89–95. - PubMed
-
- Marshall JC, Reinhart K. Biomarkers of sepsis. Crit Care Med. 2009;37(7):2290–2298. - PubMed
-
- Puntmann VO. How-to guide on biomarkers: biomarker definitions, validation and applications with examples from cardiovascular disease. Postgrad Med J. 2009;85(1008):538–545. - PubMed
-
- Ridker PM, Rifai N, Rose L, Buring JE, Cook NR. Comparison of C-reactive protein and low-density lipoprotein cholesterol levels in the prediction of first cardiovascular events. N Engl J Med. 2002;347(20):1557–1565. - PubMed
-
- Ridker PM, Cannon CP, Morrow D, Rifai N, Rose LM, McCabe CH, Pfeffer MA, Braunwald E. C-reactive protein levels and outcomes after statin therapy. N Engl J Med. 2005;352(1):20–28. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

