ERK activation drives intestinal tumorigenesis in Apc(min/+) mice
- PMID: 20473309
- PMCID: PMC2882530
- DOI: 10.1038/nm.2143
ERK activation drives intestinal tumorigenesis in Apc(min/+) mice
Abstract
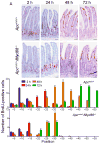
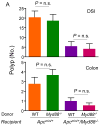
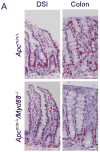
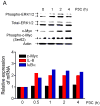
Toll-like receptor (TLR) signaling is essential for intestinal tumorigenesis in Apc(min/+) mice, but the mechanisms by which Apc enhances tumor growth are unknown. Here we show that microflora-MyD88-ERK signaling in intestinal epithelial cells (IECs) promotes tumorigenesis by increasing the stability of the c-Myc oncoprotein. Activation of ERK (extracellular signal-related kinase) phosphorylates c-Myc, preventing its ubiquitination and subsequent proteasomal degradation. Accordingly, Apc(min/+)/Myd88(-/-) mice have lower phospho-ERK (p-ERK) levels and fewer and smaller IEC tumors than Apc(min/+) mice. MyD88 (myeloid differentiation primary response gene 88)-independent activation of ERK by epidermal growth factor (EGF) increased p-ERK and c-Myc and restored the multiple intestinal neoplasia (Min) phenotype in Apc(min/+)/Myd88(-/-) mice. Administration of an ERK inhibitor suppressed intestinal tumorigenesis in EGF-treated Apc(min/+)/Myd88(-/-) and Apc(min/+) mice and increased their survival. Our data reveal a new facet of oncogene-environment interaction, in which microflora-induced TLR activation regulates oncogene expression and related IEC tumor growth in a susceptible host.
Conflict of interest statement
The authors declare that they have no competing financial interest.
Figures







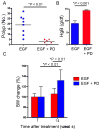

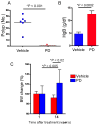



Comment in
-
Microflora in colorectal cancer: a friend to fear.Nat Med. 2010 Jun;16(6):639-41. doi: 10.1038/nm0610-639. Nat Med. 2010. PMID: 20526315 No abstract available.
References
-
- Michelsen KS, Arditi M. Toll-like receptors and innate immunity in gut homeostasis and pathology. Curr Opin Hematol. 2007;14:48–54. - PubMed
-
- de Visser KE, Eichten A, Coussens LM. Paradoxical roles of the immune system during cancer development. Nat Rev Cancer. 2006;6:24–37. - PubMed
-
- Karin M. Nuclear factor-kappaB in cancer development and progression. Nature. 2006;441:431–436. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

