Caffeine and 2-Aminoethoxydiphenyl Borate (2-APB) Have Different Ability to Inhibit Intracellular Calcium Mobilization in Pancreatic Acinar Cell
- PMID: 20473382
- PMCID: PMC2869459
- DOI: 10.4196/kjpp.2010.14.2.105
Caffeine and 2-Aminoethoxydiphenyl Borate (2-APB) Have Different Ability to Inhibit Intracellular Calcium Mobilization in Pancreatic Acinar Cell
Abstract
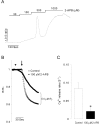
Inositol 1,4,5-trisphosphate receptors (InsP(3)Rs) modulate Ca(2+) release from intracellular Ca(2+) store and are extensively expressed in the membrane of endoplasmic/sarcoplasmic reticulum and Golgi. Although caffeine and 2-aminoethoxydiphenyl borate (2-APB) have been widely used to block InsP(3)Rs, the use of these is limited due to their multiple actions. In the present study, we examined and compared the ability of caffeine and 2-APB as a blocker of Ca(2+) release from intracellular Ca(2+) stores and Ca(2+) entry through store-operated Ca(2+) (SOC) channel in the mouse pancreatic acinar cell. Caffeine did not block the Ca(2+) entry, but significantly inhibited carbamylcholine (CCh)-induced Ca(2+) release. In contrast, 2-APB did not block CCh-induced Ca(2+) release, but remarkably blocked SOC-mediated Ca(2+) entry at lower concentrations. In permeabilized acinar cell, caffeine had an inhibitory effect on InsP(3)-induced Ca(2+) release, but 2-APB at lower concentration, which effectively blocked Ca(2+) entry, had no inhibitory action. At higher concentrations, 2-APB has multiple paradoxical effects including inhibition of InsP(3)-induced Ca(2+) release and direct stimulation of Ca(2+) release. Based on the results, we concluded that caffeine is useful as an inhibitor of InsP(3)R, and 2-APB at lower concentration is considered a blocker of Ca(2+) entry through SOC channels in the pancreatic acinar cell.
Keywords: 2-APB; Acinar; Caffeine; InsP3R; SOC.
Figures




References
-
- Berridge MJ, Bootman MD, Roderick HL. Calcium signalling: dynamics, homeostasis and remodelling. Nat Rev Mol Cell Biol. 2003;4:517–529. - PubMed
-
- Petersen OH, Michalak M, Verkhratsky A. Calcium signalling: past, present and future. Cell Calcium. 2005;38:161–169. - PubMed
-
- Williams JA, Yule DI. Stimulus-secretion coupling in pancreatic acinar cell. In: Johnson LR, editor. Physiology of the gastrointestinal tract. 4th ed. New York: Elsevier Academic Press; 2006. pp. 1337–1369.
-
- Berridge MJ. Inositol trisphosphate and calcium signalling mechanisms. Biochim Biophys Acta. 2009;1793:933–940. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

