CFTR chloride channel in the apical compartments: spatiotemporal coupling to its interacting partners
- PMID: 20473396
- PMCID: PMC2989726
- DOI: 10.1039/b924455g
CFTR chloride channel in the apical compartments: spatiotemporal coupling to its interacting partners
Abstract
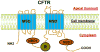
The cystic fibrosis transmembrane conductance regulator (CFTR) is a cAMP-regulated chloride channel located primarily at the apical or luminal surfaces of epithelial cells in the airway, intestine, pancreas, kidney, sweat gland, as well as male reproductive tract, where it plays a crucial role in transepithelial fluid homeostasis. CFTR dysfunction can be detrimental and may result in life-threatening disorders. CFTR hypofunctioning because of genetic defects leads to cystic fibrosis, the most common lethal genetic disease in Caucasians, whereas CFTR hyperfunctioning resulting from various infections evokes secretory diarrhea, the leading cause of mortality in early childhood. Therefore, maintaining a dynamic balance between CFTR up-regulating processes and CFTR down-regulating processes is essential for maintaining fluid and body homeostasis. Accumulating evidence suggests that protein-protein interactions play a critical role in the fine-tuned regulation of CFTR function. A growing number of proteins have been reported to interact directly or indirectly with CFTR chloride channel, suggesting that CFTR might be coupled spatially and temporally to a wide variety of interacting partners including ion channels, receptors, transporters, scaffolding proteins, enzyme molecules, signaling molecules, and effectors. Most interactions occur primarily between the opposing terminal tails (amino or carboxyl) of CFTR protein and its binding partners, either directly or mediated through various PDZ scaffolding proteins. These dynamic interactions impact the channel function, as well as localization and processing of CFTR protein within cells. This article reviews the most recent progress and findings about the interactions between CFTR and its binding partners through PDZ scaffolding proteins, as well as the spatiotemporal regulation of CFTR-containing macromolecular signaling complexes in the apical compartments of polarized cells lining the secretory epithelia.
Figures





References
-
- Welsh MJ, Tsui L-C, Boat TF, Beaudet AL. In: The Metabolic and Molecular Basis of Inherited Diseases: Membrane Transport Systems. Scriver C, Beaudet AL, Sly WS, Valle D, editors. McGraw-Hill; New York, NY: 1995. pp. 3799–3876.
-
- Dean M, Rzhetsky A, Allikmets R. Genome Res. 2001;11:1156–1166. - PubMed
-
- Riordan JR, Rommens JM, Kerem B, Alon N, Rozmahel R, Grzelczak Z, Zielenski J, Lok S, Plavsic N, Chou JL, et al. Science. 1989;245:1066–1073. - PubMed
-
- Li C, Naren AP. Pharmacol Ther. 2005;108:208–223. - PubMed
-
- Cormet-Boyaka E, Naren AP, Kirk KL. In: The Cystic Fibrosis Transmembrane Conductance Regulator. Kirk KL, Dawson DC, editors. Kluwer Academic/Plenum Publishers; New York, NY: 2003. pp. 94–118.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

