Effects of oxygen, growth state, and senescence on the antioxidant responses of WI-38 fibroblasts
- PMID: 20473639
- PMCID: PMC2980593
- DOI: 10.1007/s11357-010-9149-5
Effects of oxygen, growth state, and senescence on the antioxidant responses of WI-38 fibroblasts
Abstract
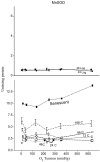
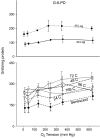
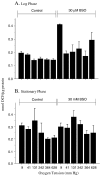
Mitotically active, growth-arrested cells and proliferatively senescent cultures of human fetal lung fibroblasts (WI-38) were exposed to six different oxygen tensions for various lengths of time and then analyzed to determine the responses of their antioxidant defense system. Glutathione (GSH) concentration increased as a function of ambient oxygen tension in early passage cultures; the effect was larger in exponentially growing cultures than in those in a state of contact-inhibited growth arrest, but was absent in senescent cells. Conversely, the activity of glutathione disulfide reductase was greater in growth-arrested cultures than in mitotically active cells irrespective of oxygen tension. Glucose-6-phosphate dehydrogenase was lowest in log-phase cells exposed to different oxygen tensions for 24 h and in senescent cells. Both hypoxia and hyperoxia depressed selenium-dependent glutathione peroxidase activity in early passage cultures, while the activity of the enzyme progressively declined with increasing oxygen in senescent cells. The GSH S-transferase activity was unresponsive to changes in ambient oxygen tension in either young or senescent cultures. Manganese-containing superoxide dismutase (MnSOD) activity was unaffected by oxygen tension, but was elevated in young confluent cultures as compared with cultures in log-phase growth. MnSOD activity was significantly higher in senescent cultures than in early passage cultures and was also responsive to increased oxygen tension in senescent cultures. Copper-zinc-containing superoxide dismutases activity was not affected by oxygen tension or the passage of time, but it declined in senescent cultures.
Figures



References
-
- Allen RG, Balin AK. Metabolic rate, free radicals and aging. In: Cutler RG, Rodriguez H, editors. Metabolic rate free radicals and aging: advances in basic science, diagnostics, and intervention. New York: World Scientific; 2003a. pp. 3–23.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
