α-Helix peptides designed from EBV-gH protein display higher antigenicity and induction of monocyte apoptosis than the native peptide
- PMID: 20473772
- PMCID: PMC2963735
- DOI: 10.1007/s00726-010-0620-5
α-Helix peptides designed from EBV-gH protein display higher antigenicity and induction of monocyte apoptosis than the native peptide
Abstract
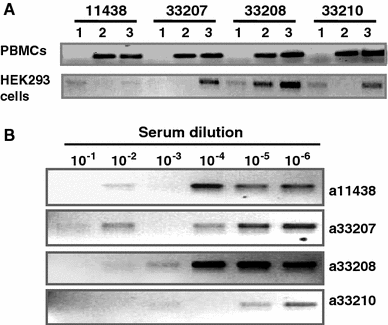
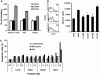
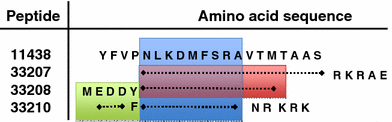
We tested the hypothesis that stabilizing α-helix of Epstein-Barr virus gH-derived peptide 11438 used for binding human cells will increase its biological activity. Non-stable α-helix of peptide 11438 was unfolded in an entropy-driven process, despite the opposing effect of the enthalpy factor. Adding and/or changing amino acids in peptide 11438 allowed the designing of peptides 33207, 33208 and 33210; peptides 33208 and 33210 displayed higher helical content due to a decreased unfolding entropy change as was determined by AGADIR, molecular dynamics and circular dichroism analysis. Peptides 33207, 33208 and 33210 inhibited EBV invasion of peripheral blood mononuclear cells and displayed epitopes more similar to native protein than peptide 11438; these peptides could be useful for detecting antibodies induced by native gH protein since they displayed high reactivity with anti-EBV antibodies. Anti-peptide 33207 antibodies showed higher reactivity with EBV than anti-peptide 11438 antibodies being useful for inducing antibodies against EBV. Anti-peptide 33210 antibodies inhibit EBV invasion of epithelial cells better than anti-peptide 11438 antibodies. Peptide 33210 bound to normal T lymphocytes and Raji cells stronger than peptide 11438 and also induced apoptosis of monocytes and Raji cells but not of normal T cells in a similar way to EBV-gH. Peptide 33210 inhibited the monocytes' development toward dendritic cells better than EBV and peptide 11438. In conclusion, stabilizing the α-helix in peptides 33208 and 33210 designed from peptide 11438 increased the antigenicity and the ability of the antibodies induced by peptides of inhibiting EBV invasion of host cells.
Figures
References
-
- Bax A, Davis DG. MLEV-17-based two-dimensional homonuclear magnetization transfer spectroscopy. J Magn Reson. 1985;65:355–360.
-
- Delves PJ. Antibody production: essential techniques. Chichester, NY: Wiley and Sons; 1997.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

