The trifunctional antibody catumaxomab for the treatment of malignant ascites due to epithelial cancer: Results of a prospective randomized phase II/III trial
- PMID: 20473913
- PMCID: PMC2958458
- DOI: 10.1002/ijc.25423
The trifunctional antibody catumaxomab for the treatment of malignant ascites due to epithelial cancer: Results of a prospective randomized phase II/III trial
Abstract
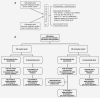
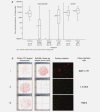
Malignant ascites is a common manifestation of advanced cancers, and treatment options are limited. The trifunctional antibody catumaxomab (anti-epithelial cell-adhesion molecule x anti-CD3) represents a targeted immunotherapy for the intraperitoneal (i.p.) treatment of malignant ascites secondary to epithelial cancers. In this phase II/III trial (EudraCT 2004-000723-15; NCT00836654), cancer patients (n = 258) with recurrent symptomatic malignant ascites resistant to conventional chemotherapy were randomized to paracentesis plus catumaxomab (catumaxomab) or paracentesis alone (control) and stratified by cancer type (129 ovarian and 129 nonovarian). Catumaxomab was administered as an i.p. infusion on Days 0, 3, 7 and 10 at doses of 10, 20, 50 and 150 mug, respectively. The primary efficacy endpoint was puncture-free survival. Secondary efficacy parameters included time to next paracentesis, ascites signs and symptoms and overall survival (OS). Puncture-free survival was significantly longer in the catumaxomab group (median 46 days) than the control group (median 11 days) (hazard ratio = 0.254: p < 0.0001) as was median time to next paracentesis (77 versus 13 days; p < 0.0001). In addition, catumaxomab patients had fewer signs and symptoms of ascites than control patients. OS showed a positive trend for the catumaxomab group and, in a prospectively planned analysis, was significantly prolonged in patients with gastric cancer (n = 66; 71 versus 44 days; p = 0.0313). Although adverse events associated with catumaxomab were frequent, they were manageable, generally reversible and mainly related to its immunologic mode of action. Catumaxomab showed a clear clinical benefit in patients with malignant ascites secondary to epithelial cancers, especially gastric cancer, with an acceptable safety profile.
Figures

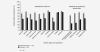
References
-
- Parsons SL, Watson SA, Steele RJC. Malignant ascites. Br J Surg. 1996;83:6–14. - PubMed
-
- Muir JC. Ascites. In: Von Roenn J, Smith TJ, Loprinzi CL, Von Gunten CF, editors. ASCO curriculum: optimizing cancer care. The importance of symptom management. Vol. 1. Dubuque, IA: Kendall/Hunt; 2001. pp. 1–31.
-
- Ayantunde AA, Parsons S. Pattern and prognostic factors in patients with malignant ascites: a retrospective study. Ann Oncol. 2007;18:945–9. - PubMed
-
- Tamsma J. The pathogenesis of malignant ascites. Cancer Treat Res. 2007;134:109–18. - PubMed
-
- Preston N. New strategies for the management of malignant ascites. Eur J Cancer Care. 1995;4:178–83. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

