Mechanical properties of cellularly responsive hydrogels and their experimental determination
- PMID: 20473984
- PMCID: PMC3890982
- DOI: 10.1002/adma.200904179
Mechanical properties of cellularly responsive hydrogels and their experimental determination
Abstract
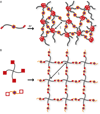
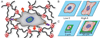
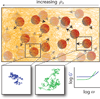
Hydrogels are increasingly employed as multidimensional cell culture platforms often with a necessity that they respond to or control the cellular environment. Specifically, synthetic hydrogels, such as poly(ethylene glycol) (PEG)-based gels, are frequently utilized for probing the microenvironment's influence on cell function, as the gel properties can be precisely controlled in space and time. Synthetically tunable parameters, such as monomer structure and concentration, facilitate initial gel property control, while incorporation of responsive degradable units enables cell- and/or user-directed degradation. Such responsive gel systems are complex with dynamic changes occurring over multiple time-scales, and cells encapsulated in these synthetic hydrogels often experience and dictate local property changes profoundly different from those in the bulk material. Consequently, advances in bulk and local measurement techniques are needed to monitor property evolution quantatively and understand its effect on cell function. Here, recent progress in cell-responsive PEG hydrogel synthesis and mechanical property characterization is reviewed.
Figures


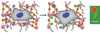


Similar articles
-
Encapsulation of primary salivary gland cells in enzymatically degradable poly(ethylene glycol) hydrogels promotes acinar cell characteristics.Acta Biomater. 2017 Mar 1;50:437-449. doi: 10.1016/j.actbio.2016.12.049. Epub 2016 Dec 27. Acta Biomater. 2017. PMID: 28039063 Free PMC article.
-
Synthesis of stiffness-tunable and cell-responsive Gelatin-poly(ethylene glycol) hydrogel for three-dimensional cell encapsulation.J Biomed Mater Res A. 2016 Oct;104(10):2401-11. doi: 10.1002/jbm.a.35779. Epub 2016 May 30. J Biomed Mater Res A. 2016. PMID: 27170015
-
Dynamic loading stimulates chondrocyte biosynthesis when encapsulated in charged hydrogels prepared from poly(ethylene glycol) and chondroitin sulfate.Matrix Biol. 2010 Jan;29(1):51-62. doi: 10.1016/j.matbio.2009.08.004. Epub 2009 Aug 29. Matrix Biol. 2010. PMID: 19720146 Free PMC article.
-
Polyethylene glycol (PEG)-Poly(N-isopropylacrylamide) (PNIPAAm) based thermosensitive injectable hydrogels for biomedical applications.Eur J Pharm Biopharm. 2014 Nov;88(3):575-85. doi: 10.1016/j.ejpb.2014.07.005. Epub 2014 Aug 1. Eur J Pharm Biopharm. 2014. PMID: 25092423 Review.
-
Poly(lactic acid) based hydrogels.Adv Drug Deliv Rev. 2016 Dec 15;107:192-205. doi: 10.1016/j.addr.2016.07.004. Epub 2016 Jul 16. Adv Drug Deliv Rev. 2016. PMID: 27432797 Review.
Cited by
-
Matrix metalloproteinase responsive hydrogel microplates for programmed killing of invasive tumour cells.RSC Appl Polym. 2023 Aug 14;1(1):19-29. doi: 10.1039/d3lp00057e. eCollection 2023 Sep 25. RSC Appl Polym. 2023. PMID: 38013908 Free PMC article.
-
Cartilage-like mechanical properties of poly (ethylene glycol)-diacrylate hydrogels.Biomaterials. 2012 Oct;33(28):6682-90. doi: 10.1016/j.biomaterials.2012.06.005. Epub 2012 Jun 30. Biomaterials. 2012. PMID: 22749448 Free PMC article.
-
2PP-Hydrogel Covered Electrodes to Compensate for Media Effects in the Determination of Biomass in a Capillary Wave Micro Bioreactor.Biosensors (Basel). 2024 Sep 9;14(9):438. doi: 10.3390/bios14090438. Biosensors (Basel). 2024. PMID: 39329813 Free PMC article.
-
Non-destructive mechanical assessment for optimization of 3D bioprinted soft tissue scaffolds.iScience. 2022 Apr 13;25(5):104251. doi: 10.1016/j.isci.2022.104251. eCollection 2022 May 20. iScience. 2022. PMID: 35521534 Free PMC article.
-
A Novel Toolkit for Characterizing the Mechanical and Electrical Properties of Engineered Neural Tissues.Biosensors (Basel). 2019 Apr 1;9(2):51. doi: 10.3390/bios9020051. Biosensors (Basel). 2019. PMID: 30939804 Free PMC article.
References
-
- Ghosh K, Ingber DE. Adv. Drug Deliv. Rev. 2007;59:1306–1318. - PubMed
-
- Adeloew C, Segura T, Hubbell JA, Frey P. Biomaterials. 2008;29:314–326. - PubMed
-
- Silva GA, Czeisler C, Niece KL, Beniash E, Harrington DA, Kessler JA, Stupp SI. Science. 2004;303:1352–1355. - PubMed
-
- Capito RM, Azevedo HS, Velichko YS, Mata A, Stupp SI. Science. 2008;319:1812–1816. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

