The stretching frequencies of bound alkyl isocyanides indicate two distinct ligand orientations within the distal pocket of myoglobin
- PMID: 20476740
- PMCID: PMC4102131
- DOI: 10.1021/bi100172c
The stretching frequencies of bound alkyl isocyanides indicate two distinct ligand orientations within the distal pocket of myoglobin
Abstract
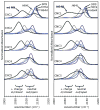
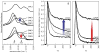
The FTIR spectra for alkyl isocyanides (CNRs) change from a single nu(CN) band centered at approximately 2175 cm(-1) to two peaks at approximately 2075 and approximately 2125 cm(-1) upon binding to sperm whale myoglobin (Mb). The low- and high-frequency peaks have been assigned to in and out conformations, respectively. In the in conformation, the ligand is pointing toward the protein interior, and the distal His64(E7) is in a closed position, donates a H-bond to the bound isocyano group, enhances back-bonding, and lowers the C-N bond order. In the out conformation, the ligand side chain points toward solvent through a channel opened by outward rotation of His64. Loss of positive polarity near the binding site causes an increase in C-N bond order. Support for this interpretation is threefold: (1) similar shifts to lower frequency occur for MbCO complexes when H-bond donation from His64(E7) occurs; (2) only one peak at approximately 2125 cm(-1), indicative of an apolar environment, is observed for CNRs bound to H64A or H64L Mb mutants or to chelated protoheme in soap micelles; and (3) the fraction of in conformation based on FTIR spectra correlates strongly with the fraction of geminate recombination after nanosecond laser photolysis. The in alkyl side chain conformation causes the photodissociated ligand to be "stuck" in the distal pocket, promoting internal rebinding, whereas the out conformation inhibits geminate recombination because part of the ligand is already in an open E7 channel, poised for rapid escape.
Figures



References
-
- St George RC, Pauling L. The combining power of hemoglobin for alkyl isocyanides, and the nature of the heme-heme interactions in hemoglobin. Science. 1951;114:629–634. - PubMed
-
- Talbot B, Brunori M, Antonini E, Wyman J. Studies on the reaction of isocyanides with haemproteins. I. Equilibria and kinetics of the binding to the isolated chains of human haemoglobin. J Mol Biol. 1971;58:261–276. - PubMed
-
- Brunori M, Talbot B, Colosimo A, Antonini E, Wyman J. Studies on the reaction of isocyanides with haemproteins. II. Binding to normal and modified human haemoglobins. J Mol Biol. 1972;65:423–434. - PubMed
-
- Stetzkowski F, Cassoly R, Banerjee R. Binding of alkylisocyanides with soybean leghemoglobin. Comparisons with sperm whale myoglobin. J Biol Chem. 1979;254:11351–11356. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

