Alkyl isocyanides serve as transition state analogues for ligand entry and exit in myoglobin
- PMID: 20476741
- PMCID: PMC4102008
- DOI: 10.1021/bi1001745
Alkyl isocyanides serve as transition state analogues for ligand entry and exit in myoglobin
Abstract
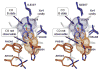
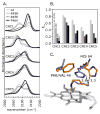
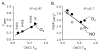
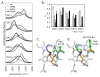
Alkyl isocyanides (CNRs) identify pathways for diatomic ligand movement into and out of Mb, with their side chains acting as transition state analogues. The bound alkyl groups point either into the back of the distal pocket (in conformation, nu(CN) approximately 2070-2090 cm(-1)), which allows hydrogen bond donation from His64(E7) to the isocyano group, or toward solvent through an open His(E7) channel (out conformation, nu(CN) approximately 2110-2130 cm(-1)), which prevents polar interactions with the isocyano atoms. Fractions of the in conformer (F(in)) were measured by FTIR spectroscopy for methyl through n-pentyl isocyanide bound to a series of 20 different distal pocket mutants of sperm whale myoglobin and found to be governed by the ease of rotation of the His(E7) side chain, distal pocket volume and steric interactions, and, for the longer isocyanides, the unfavorable hydrophobic effect of placing their terminal carbon atoms into the solvent phase in the out conformation. There are strong correlations between the fraction of in conformer, F(in), for long-chain MbCNR complexes measured by FTIR spectroscopy, the fraction of geminate recombination of photodissociated O(2), and the bimolecular rates of O(2) entry into the distal pocket. These correlations indicate that alkyl isocyanides serve as transition state analogues for the movement of O(2) into and out of the binding pocket of Mb.
Figures




References
-
- Scott EE, Gibson QH. Ligand migration in sperm whale myoglobin. Biochemistry. 1997;36:11909–11917. - PubMed
-
- Scott EE, Gibson QH, Olson JS. Mapping the pathways for O2 entry into and exit from myoglobin. J Biol Chem. 2001;276:5177–5188. - PubMed
-
- Olson JS, Soman J, Phillips GN., Jr Ligand pathways in myoglobin: a review of Trp cavity mutations. IUBMB Life. 2007;59:552–562. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

