Conformational selection in the recognition of the snurportin importin beta binding domain by importin beta
- PMID: 20476751
- PMCID: PMC6013741
- DOI: 10.1021/bi100292y
Conformational selection in the recognition of the snurportin importin beta binding domain by importin beta
Abstract
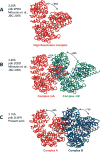
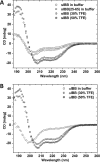
The structural flexibility of beta-karyopherins is critical to mediate the interaction with transport substrates, nucleoporins, and the GTPase Ran. In this paper, we provide structural evidence that the molecular recognition of the transport adaptor snurportin by importin beta follows the population selection mechanism. We have captured two drastically different conformations of importin beta bound to the snurportin importin beta binding domain trapped in the same crystallographic asymmetric unit. We propose the population selection may be a general mechanism used by beta-karyopherins to recognize transport substrates.
Figures


References
-
- Stewart M. Molecular mechanism of the nuclear protein import cycle. Nat. Rev. Mol. Cell Biol. 2007;8:195–208. - PubMed
-
- Cook A, Bono F, Jinek M, Conti E. Structural biology of nucleocytoplasmic transport. Annu. Rev. Biochem. 2007;76:647–671. - PubMed
-
- Cingolani G, Bednenko J, Gillespie MT, Gerace L. Molecular basis for the recognition of a nonclassical nuclear localization signal by importin β. Mol. Cell. 2002;10:1345–1353. - PubMed
-
- Cingolani G, Petosa C, Weis K, Muller CW. Structure of importin-β bound to the IBB domain of importin-α. Nature. 1999;399:221–229. - PubMed
-
- Lee SJ, Sekimoto T, Yamashita E, Nagoshi E, Nakagawa A, Imamoto N, Yoshimura M, Sakai H, Chong KT, Tsukihara T, Yoneda Y. The structure of importin-β bound to SREBP-2: Nuclear import of a transcription factor. Science. 2003;302:1571–1575. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

