Increasing the conformational entropy of the Omega-loop lid domain in phosphoenolpyruvate carboxykinase impairs catalysis and decreases catalytic fidelity
- PMID: 20476774
- PMCID: PMC2893389
- DOI: 10.1021/bi100399e
Increasing the conformational entropy of the Omega-loop lid domain in phosphoenolpyruvate carboxykinase impairs catalysis and decreases catalytic fidelity
Abstract
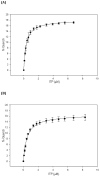
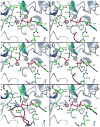
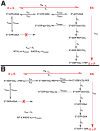
Many studies have shown that the dynamic motions of individual protein segments can play an important role in enzyme function. Recent structural studies of the gluconeogenic enzyme phosphoenolpyruvate carboxykinase (PEPCK) demonstrate that PEPCK contains a 10-residue Omega-loop domain that acts as an active site lid. On the basis of these structural studies, we have previously proposed a model for the mechanism of PEPCK catalysis in which the conformation of this mobile lid domain is energetically coupled to ligand binding, resulting in the closed conformation of the lid, necessary for correct substrate positioning, becoming more energetically favorable as ligands associate with the enzyme. Here we test this model by introducing a point mutation (A467G) into the center of the Omega-loop lid that is designed to increase the entropic penalty for lid closure. Structural and kinetic characterization of this mutant enzyme demonstrates that the mutation has decreased the favorability of the enzyme adapting the closed lid conformation. As a consequence of this shift in the equilibrium defining the conformation of the active site lid, the enzyme's ability to stabilize the reaction intermediate is weakened, resulting in catalytic defect. This stabilization is initially surprising, as the lid domain makes no direct contacts with the enolate intermediate formed during the reaction. Furthermore, during the conversion of OAA to PEP, the destabilization of the lid-closed conformation results in the reaction becoming decoupled as the enolate intermediate is protonated rather than phosphorylated, resulting in the formation of pyruvate. Taken together, the structural and kinetic characterization of A467G-PEPCK supports our model of the role of the active site lid in catalytic function and demonstrates that the shift in the lowest-energy conformation between open and closed lid states is a function of the free energy available to the enzyme through ligand binding and the entropic penalty for ordering of the 10-residue Omega-loop lid domain.
Figures






References
-
- Lee MH, Hebda CA, Nowak T. The Role of Cations in Avian Liver Phosphoenolpyruvate Carboxykinase Catalysis. Activation and Regulation. J Biol Chem. 1981;256:12793–12801. - PubMed
-
- Colombo G, Carlson GM, Lardy HA. Phosphoenolpyruvate Carboxykinase (Guanosine 5′-Triphosphate) from Rat Liver Cytosol. Dual-Cation Requirement for the Carboxylation Reaction. Biochemistry. 1981;20:2749–2757. - PubMed
-
- Hebda CA, Nowak T. The Purification, Characterization, and Activation of Phosphoenolpyruvate Carboxykinase from Chicken Liver Mitochondria. J Biol Chem. 1982;257:5503–5514. - PubMed
-
- Hebda CA, Nowak T. Phosphoenolpyruvate Carboxykinase. Mn2+ and Mn2+ Substrate Complexes. J Biol Chem. 1982;257:5515–5522. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

