AMP-activated protein kinase deficiency exacerbates aging-induced myocardial contractile dysfunction
- PMID: 20477759
- PMCID: PMC2910211
- DOI: 10.1111/j.1474-9726.2010.00586.x
AMP-activated protein kinase deficiency exacerbates aging-induced myocardial contractile dysfunction
Abstract
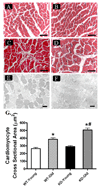
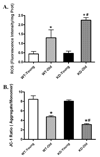
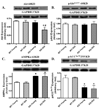
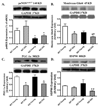
Aging is associated with myocardial dysfunction although the underlying mechanism is unclear. AMPK, a key cellular fuel sensor for energy metabolism, is compromised with aging. This study examined the role of AMPK deficiency in aging-associated myocardial dysfunction. Young or old wild-type (WT) and transgenic mice with overexpression of a mutant AMPK alpha(2) subunit (kinase dead, KD) were used. AMPK alpha isoform activity, myocardial function and morphology were examined. DCF and JC-1 fluorescence probes were employed to quantify reactive oxygen species (ROS) and mitochondrial membrane potential (DeltaPsim), respectively. KD mice displayed significantly reduced alpha(2) but not alpha(1) AMPK isoform activity at both ages with a greater effect at old age. Aging itself decreased alpha(1) isoform activity. Cardiomyocyte contractile function, intracellular Ca(2+) handling, and SERCA2a levels were compromised with aging, the effects of which were exacerbated by AMPK deficiency. H&E staining revealed cardiomyocyte hypertrophy with aging, which was more pronounced in KD mice. TEM micrographs displayed severe disruption of mitochondrial ultrastructure characterized by swollen, irregular shape and disrupted cristae in aged KD compared with WT mice. Aging enhanced ROS production and reduced DeltaPsim, the effects of which were accentuated by AMPK deficiency. Immunoblotting data depicted unchanged Akt phosphorylation and a significant decrease in mitochondrial biogenesis cofactor PGC-1alpha in aged groups. AMPK deficiency but not aging decreased the phosphorylation of ACC and eNOS. Expression of membrane Glut4 and HSP90 was decreased in aged KD mice. Moreover, treatment of the AMPK activator metformin attenuated aging-induced cardiomyocyte contractile defects. Collectively, our data suggest a role for AMPK deficiency in aging-induced cardiac dysfunction possibly through disrupted mitochondrial function and ROS production.
Figures






References
-
- Arad M, Seidman CE, Seidman JG. AMP-Activated Protein Kinase in the Heart: Role During Health and Disease. Circ Res. 2007;100:474–488. - PubMed
-
- Bhashyam S, Parikh P, Bolukoglu H, Shannon AH, Porter JH, Shen YT, Shannon RP. Aging Is Associated With Myocardial Insulin Resistance and Mitochondrial Dysfunction. Am J Physiol Heart Circ Physiol. 2007;293:H3063–H3071. - PubMed
-
- Calvert JW, Gundewar S, Jha S, Greer JJ, Bestermann WH, Tian R, Lefer DJ. Acute Metformin Therapy Confers Cardioprotection Against Myocardial Infarction Via AMPK-ENOS-Mediated Signaling. Diabetes. 2008;57:696–705. - PubMed
-
- Chan AY, Soltys CL, Young ME, Proud CG, Dyck JR. Activation of AMP-Activated Protein Kinase Inhibits Protein Synthesis Associated With Hypertrophy in the Cardiac Myocyte. J Biol Chem. 2004;279:32771–32779. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

