Mechanosensitive hair cell-like cells from embryonic and induced pluripotent stem cells
- PMID: 20478259
- PMCID: PMC2873974
- DOI: 10.1016/j.cell.2010.03.035
Mechanosensitive hair cell-like cells from embryonic and induced pluripotent stem cells
Abstract
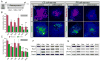
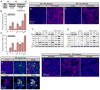
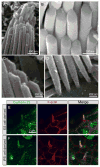
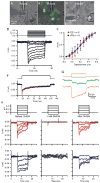
Mechanosensitive sensory hair cells are the linchpin of our senses of hearing and balance. The inability of the mammalian inner ear to regenerate lost hair cells is the major reason for the permanence of hearing loss and certain balance disorders. Here, we present a stepwise guidance protocol starting with mouse embryonic stem and induced pluripotent stem cells, which were directed toward becoming ectoderm capable of responding to otic-inducing growth factors. The resulting otic progenitor cells were subjected to varying differentiation conditions, one of which promoted the organization of the cells into epithelial clusters displaying hair cell-like cells with stereociliary bundles. Bundle-bearing cells in these clusters responded to mechanical stimulation with currents that were reminiscent of immature hair cell transduction currents.
Copyright (c) 2010 Elsevier Inc. All rights reserved.
Figures


Similar articles
-
Inner ear hair cell-like cells from human embryonic stem cells.Stem Cells Dev. 2014 Jun 1;23(11):1275-84. doi: 10.1089/scd.2014.0033. Epub 2014 Mar 10. Stem Cells Dev. 2014. PMID: 24512547 Free PMC article.
-
Generating inner ear organoids containing putative cochlear hair cells from human pluripotent stem cells.Cell Death Dis. 2018 Sep 11;9(9):922. doi: 10.1038/s41419-018-0967-1. Cell Death Dis. 2018. PMID: 30206231 Free PMC article.
-
Directed Differentiation of Mouse Embryonic Stem Cells Into Inner Ear Sensory Epithelia in 3D Culture.Methods Mol Biol. 2017;1597:67-83. doi: 10.1007/978-1-4939-6949-4_6. Methods Mol Biol. 2017. PMID: 28361311 Free PMC article.
-
Stereocilia Rootlets: Actin-Based Structures That Are Essential for Structural Stability of the Hair Bundle.Int J Mol Sci. 2020 Jan 3;21(1):324. doi: 10.3390/ijms21010324. Int J Mol Sci. 2020. PMID: 31947734 Free PMC article. Review.
-
Hair cell regeneration in the inner ear.Otolaryngol Head Neck Surg. 1994 Sep;111(3 Pt 1):281-301. doi: 10.1177/01945998941113P118. Otolaryngol Head Neck Surg. 1994. PMID: 8084636 Review.
Cited by
-
Stem cell therapy for the inner ear: recent advances and future directions.Trends Amplif. 2012 Mar;16(1):4-18. doi: 10.1177/1084713812440336. Epub 2012 Apr 17. Trends Amplif. 2012. PMID: 22514095 Free PMC article. Review.
-
N-Myc and L-Myc are essential for hair cell formation but not maintenance.Brain Res. 2012 Nov 12;1484:1-14. doi: 10.1016/j.brainres.2012.09.027. Epub 2012 Sep 25. Brain Res. 2012. PMID: 23022312 Free PMC article.
-
Early steps in inner ear development: induction and morphogenesis of the otic placode.Front Pharmacol. 2015 Feb 10;6:19. doi: 10.3389/fphar.2015.00019. eCollection 2015. Front Pharmacol. 2015. PMID: 25713536 Free PMC article. Review.
-
Neurod1 suppresses hair cell differentiation in ear ganglia and regulates hair cell subtype development in the cochlea.PLoS One. 2010 Jul 22;5(7):e11661. doi: 10.1371/journal.pone.0011661. PLoS One. 2010. PMID: 20661473 Free PMC article.
-
[Advances in stem cell inner ear transplantation].Lin Chuang Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2022 Mar;36(3):239-242. doi: 10.13201/j.issn.2096-7993.2022.03.017. Lin Chuang Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2022. PMID: 35193350 Free PMC article. Review. Chinese.
References
-
- Alsina B, Abello G, Ulloa E, Henrique D, Pujades C, Giraldez F. FGF signaling is required for determination of otic neuroblasts in the chick embryo. Dev Biol. 2004;267:119–134. - PubMed
-
- Azuara V, Perry P, Sauer S, Spivakov M, Jorgensen HF, John RM, Gouti M, Casanova M, Warnes G, Merkenschlager M, Fisher AG. Chromatin signatures of pluripotent cell lines. Nat Cell Biol. 2006;8:532–538. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

