A transitional extracellular matrix instructs cell behavior during muscle regeneration
- PMID: 20478295
- PMCID: PMC4157629
- DOI: 10.1016/j.ydbio.2010.05.007
A transitional extracellular matrix instructs cell behavior during muscle regeneration
Abstract
Urodele amphibians regenerate appendages through the recruitment of progenitor cells into a blastema that rebuilds the lost tissue. Blastemal formation is accompanied by extensive remodeling of the extracellular matrix. Although this remodeling process is important for appendage regeneration, it is not known whether the remodeled matrix directly influences the generation and behavior of blastemal progenitor cells. By integrating in vivo 3-dimensional spatiotemporal matrix maps with in vitro functional time-lapse imaging, we show that key components of this dynamic matrix, hyaluronic acid, tenascin-C and fibronectin, differentially direct cellular behaviors including DNA synthesis, migration, myotube fragmentation and myoblast fusion. These data indicate that both satellite cells and fragmenting myofibers contribute to the regeneration blastema and that the local extracellular environment provides instructive cues for the regenerative process. The fact that amphibian and mammalian myoblasts exhibit similar responses to various matrices suggests that the ability to sense and respond to regenerative signals is evolutionarily conserved.
Copyright (c) 2010 Elsevier Inc. All rights reserved.
Figures
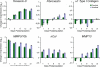
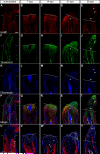
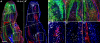

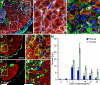
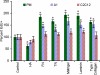
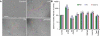


References
-
- Alvarado AS. Regeneration in the metazoans: Why does it happen? BioEssays. 2000;22:578–590. - PubMed
-
- Blau HM, Chiu CP, Webster C. Cytoplasmic activation of human nuclear genes in stable heterocaryons. Cell. 1983;32:1171–80. - PubMed
-
- Boonen KJM, Rosaria-Chak KY, Baaijens FPT, van der Schaft DWJ, Post MJ. Essential environmental cues from the satellite cell niche: Optimizing proliferation and differentiation. Am. J. Physiol.-Cell Ph. 2009;296:C1338–C1345. - PubMed
-
- Bouchentouf M, Benabdallah BF, Mills P, Tremblay JP. Exercise improves the success of myoblast transplantation in mdx mice. Neuromuscular Disord. 2006;16:518–529. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

