AMPK supports growth in Drosophila by regulating muscle activity and nutrient uptake in the gut
- PMID: 20478298
- PMCID: PMC2909368
- DOI: 10.1016/j.ydbio.2010.05.010
AMPK supports growth in Drosophila by regulating muscle activity and nutrient uptake in the gut
Abstract
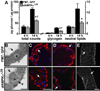
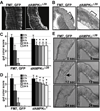
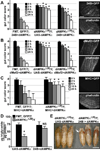
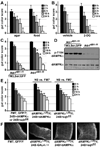
The larval phase of the Drosophila life cycle is characterized by constant food intake, resulting in a two hundred-fold increase in mass over four days. Here we show that the conserved energy sensor AMPK is essential for nutrient intake in Drosophila. Mutants lacking dAMPKalpha are small, with low triglyceride levels, small fat body cells and early pupal lethality. Using mosaic analysis, we find that dAMPKalpha functions as a nonautonomous regulator of cell growth. Nutrient absorption is impaired in dAMPKalpha mutants, and this defect stems not from altered gut epithelial cell polarity but from impaired peristaltic activity. Expression of a wild-type dAMPKalpha transgene or an activated version of the AMPK target myosin regulatory light chain (MRLC) in the dAMPKalpha mutant visceral musculature restores gut function and growth. These data suggest strongly that AMPK regulates visceral smooth muscle function through phosphorylation of MRLC. Furthermore, our data show that in Drosophila, AMPK performs an essential cell-nonautonomous function, serving the needs of the organism by promoting activity of the visceral musculature and, consequently, nutrient intake.
Copyright (c) 2010 Elsevier Inc. All rights reserved.
Figures


Similar articles
-
A critical role of SNF1A/dAMPKalpha (Drosophila AMP-activated protein kinase alpha) in muscle on longevity and stress resistance in Drosophila melanogaster.Biochem Biophys Res Commun. 2010 Mar 26;394(1):112-8. doi: 10.1016/j.bbrc.2010.02.126. Epub 2010 Feb 23. Biochem Biophys Res Commun. 2010. PMID: 20184862
-
Energy-dependent regulation of cell structure by AMP-activated protein kinase.Nature. 2007 Jun 21;447(7147):1017-20. doi: 10.1038/nature05828. Epub 2007 May 7. Nature. 2007. PMID: 17486097
-
Deletion of intestinal epithelial AMP-activated protein kinase alters distal colon permeability but not glucose homeostasis.Mol Metab. 2021 May;47:101183. doi: 10.1016/j.molmet.2021.101183. Epub 2021 Feb 4. Mol Metab. 2021. PMID: 33548500 Free PMC article.
-
LKB1 and AMPK in cell polarity and division.Trends Cell Biol. 2008 Apr;18(4):193-8. doi: 10.1016/j.tcb.2008.01.008. Epub 2008 Mar 7. Trends Cell Biol. 2008. PMID: 18314332 Review.
-
A critical role of AMP-activated protein kinase in regulating intestinal nutrient absorption, barrier function, and intestinal diseases.J Cell Physiol. 2022 Oct;237(10):3705-3716. doi: 10.1002/jcp.30841. Epub 2022 Jul 26. J Cell Physiol. 2022. PMID: 35892164 Review.
Cited by
-
Anatomy and Physiology of the Digestive Tract of Drosophila melanogaster.Genetics. 2018 Oct;210(2):357-396. doi: 10.1534/genetics.118.300224. Genetics. 2018. PMID: 30287514 Free PMC article. Review.
-
Necrotic Enteritis in Broiler Chickens: The Role of Tight Junctions and Mucosal Immune Responses in Alleviating the Effect of the Disease.Microorganisms. 2019 Jul 31;7(8):231. doi: 10.3390/microorganisms7080231. Microorganisms. 2019. PMID: 31370350 Free PMC article.
-
Misato underlies visceral myopathy in Drosophila.Sci Rep. 2017 Dec 18;7(1):17700. doi: 10.1038/s41598-017-17961-3. Sci Rep. 2017. PMID: 29255146 Free PMC article.
-
Muscle function and homeostasis require cytokine inhibition of AKT activity in Drosophila.Elife. 2020 Jan 20;9:e51595. doi: 10.7554/eLife.51595. Elife. 2020. PMID: 31944178 Free PMC article.
-
SAGA function in tissue-specific gene expression.Trends Cell Biol. 2012 Apr;22(4):177-84. doi: 10.1016/j.tcb.2011.11.005. Epub 2011 Dec 21. Trends Cell Biol. 2012. PMID: 22196215 Free PMC article.
References
-
- Baumann O. Posterior midgut epithelial cells differ in their organization of the membrane skeleton from other drosophila epithelia. Exp Cell Res. 2001;270:176–187. - PubMed
-
- Britton JS, Edgar BA. Environmental control of the cell cycle in Drosophila: nutrition activates mitotic and endoreplicative cells by distinct mechanisms. Development. 1998;125:2149–2158. - PubMed
-
- Britton JS, Lockwood WK, Li L, Cohen SM, Edgar BA. Drosophila's insulin/PI3-kinase pathway coordinates cellular metabolism with nutritional conditions. Dev Cell. 2002;2:239–249. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

