Analysis of early human neural crest development
- PMID: 20478300
- PMCID: PMC2927129
- DOI: 10.1016/j.ydbio.2010.05.012
Analysis of early human neural crest development
Abstract
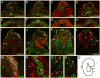
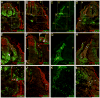
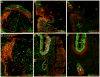
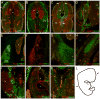
The outstanding migration and differentiation capacities of neural crest cells (NCCs) have fascinated scientists since Wilhelm His described this cell population in 1868. Today, after intense research using vertebrate model organisms, we have gained considerable knowledge regarding the origin, migration and differentiation of NCCs. However, our understanding of NCC development in human embryos remains largely uncharacterized, despite the role the neural crest plays in several human pathologies. Here, we report for the first time the expression of a battery of molecular markers before, during, or following NCC migration in human embryos from Carnegie Stages (CS) 12 to 18. Our work demonstrates the expression of Sox9, Sox10 and Pax3 transcription factors in premigratory NCCs, while actively migrating NCCs display the additional transcription factors Pax7 and AP-2alpha. Importantly, while HNK-1 labels few migrating NCCs, p75(NTR) labels a large proportion of this population. However, the broad expression of p75(NTR) - and other markers - beyond the neural crest stresses the need for the identification of additional markers to improve our capacity to investigate human NCC development, and to enable the generation of better diagnostic and therapeutic tools.
Copyright 2010 Elsevier Inc. All rights reserved.
Figures


References
-
- Abzhanov A, Tzahor E, Lassar AB, Tabin CJ. Dissimilar regulation of cell differentiation in mesencephalic (cranial) and sacral (trunk) neural crest cells in vitro. Development. 2003;130:4567–79. - PubMed
-
- Anderson RB, Stewart AL, Young HM. Phenotypes of neural-crest-derived cells in vagal and sacral pathways. Cell Tissue Res. 2006;323:11–25. - PubMed
-
- Auerbach R. Analysis of the developmental effects of a lethal mutation in the house mouse. Journal of Experimental Zoology. 1954;127:305–329.
-
- Aybar MJ, Mayor R. Early induction of neural crest cells: lessons learned from frog, fish and chick. Curr Opin Genet Dev. 2002;12:452–8. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

