Dendritic alterations after dynamic axonal stretch injury in vitro
- PMID: 20478308
- PMCID: PMC3979358
- DOI: 10.1016/j.expneurol.2010.05.001
Dendritic alterations after dynamic axonal stretch injury in vitro
Abstract
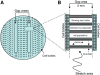
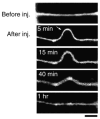
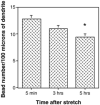
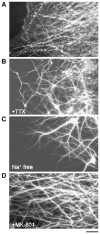
Traumatic axonal injury (TAI) is the most common and important pathology of traumatic brain injury (TBI). However, little is known about potential indirect effects of TAI on dendrites. In this study, we used a well-established in vitro model of axonal stretch injury to investigate TAI-induced changes in dendrite morphology. Axons bridging two separated rat cortical neuron populations plated on a deformable substrate were used to create a zone of isolated stretch injury to axons. Following injury, we observed the formation of dendritic alterations or beading along the dendrite shaft. Dendritic beading formed within minutes after stretch then subsided over time. Pharmacological experiments revealed a sodium-dependent mechanism, while removing extracellular calcium exacerbated TAI's effect on dendrites. In addition, blocking ionotropic glutamate receptors with the N-methyl-d-aspartate (NMDA) receptor antagonist MK-801 prevented dendritic beading. These results demonstrate that axon mechanical injury directly affects dendrite morphology, highlighting an important bystander effect of TAI. The data also imply that TAI may alter dendrite structure and plasticity in vivo. An understanding of TAI's effect on dendrites is important since proper dendrite function is crucial for normal brain function and recovery after injury.
(c) 2010. Published by Elsevier Inc.
Figures


Similar articles
-
Distinct roles for sodium, chloride, and calcium in excitotoxic dendritic injury and recovery.Exp Neurol. 1998 Nov;154(1):241-58. doi: 10.1006/exnr.1998.6929. Exp Neurol. 1998. PMID: 9875285
-
BMP-7 and excess glutamate: opposing effects on dendrite growth from cerebral cortical neurons in vitro.Exp Neurol. 2002 Jul;176(1):41-54. doi: 10.1006/exnr.2002.7906. Exp Neurol. 2002. PMID: 12093081
-
Effect of excess extracellular glutamate on dendrite growth from cerebral cortical neurons at 3 days in vitro: Involvement of NMDA receptors.J Neurosci Res. 2003 Dec 1;74(5):688-700. doi: 10.1002/jnr.10797. J Neurosci Res. 2003. PMID: 14635220
-
Changes in microtubule-associated protein 2 and amyloid precursor protein immunoreactivity following traumatic brain injury in rat: influence of MK-801 treatment.Brain Res. 1996 May 6;719(1-2):161-71. doi: 10.1016/0006-8993(96)00081-9. Brain Res. 1996. PMID: 8782876
-
Glutamate receptor agonist kainate enhances primary dendrite number and length from immature mouse cortical neurons in vitro.J Neurosci Res. 2006 May 1;83(6):944-56. doi: 10.1002/jnr.20805. J Neurosci Res. 2006. PMID: 16498632
Cited by
-
Axonal pathology in traumatic brain injury.Exp Neurol. 2013 Aug;246:35-43. doi: 10.1016/j.expneurol.2012.01.013. Epub 2012 Jan 20. Exp Neurol. 2013. PMID: 22285252 Free PMC article. Review.
-
Coupled left-shift of Nav channels: modeling the Na⁺-loading and dysfunctional excitability of damaged axons.J Comput Neurosci. 2012 Oct;33(2):301-19. doi: 10.1007/s10827-012-0387-7. Epub 2012 Apr 5. J Comput Neurosci. 2012. PMID: 22476614
-
Temporal and structural sensitivities of major biomarkers for detecting neuropathology after traumatic brain injury in the mouse.Front Neurosci. 2024 Jan 30;18:1339262. doi: 10.3389/fnins.2024.1339262. eCollection 2024. Front Neurosci. 2024. PMID: 38356651 Free PMC article.
-
Characterization of neurite dystrophy after trauma by high speed structured illumination microscopy and lattice light sheet microscopy.J Neurosci Methods. 2019 Jan 15;312:154-161. doi: 10.1016/j.jneumeth.2018.12.005. Epub 2018 Dec 6. J Neurosci Methods. 2019. PMID: 30529411 Free PMC article.
-
Microfluidic culture platform for studying neuronal response to mild to very mild axonal stretch injury.Biomicrofluidics. 2014 Jul 22;8(4):044110. doi: 10.1063/1.4891098. eCollection 2014 Jul. Biomicrofluidics. 2014. PMID: 25379095 Free PMC article.
References
-
- Al-Noori S, Swann JW. A role for sodium and chloride in kainic acid-induced beading of inhibitory interneuron dendrites. Neuroscience. 2000;101:337–348. - PubMed
-
- Briones TL, Woods J, Wadowska M, Rogozinska M. Amelioration of cognitive impairment and changes in microtubule-associated protein 2 after transient global cerebral ischemia are influenced bycomplex environment experience. Behav Brain Res. 2006;168:261–271. - PubMed
-
- Castejon OJ, Arismendi GJ. Morphological changes of dendrites in the human edematous cerebral cortex. A transmission electron microscopic study. J Submicrosc Cytol Pathol. 2003;35:395–413. - PubMed
-
- Castejon OJ, Valero C, Diaz M. Light and electron microscopy study of nerve cells in traumatic oedematous human cerebral cortex. Brain Inj. 1997;11:363–388. - PubMed
-
- Castejon OJ, Castellano A, Arismendi G. Transmission electron microscopy of cortical dendritic spines in the human oedematous cerebral cortex. J Submicrosc Cytol Pathol. 2004;36:181–191. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

