Sonic hedgehog induces angiogenesis via Rho kinase-dependent signaling in endothelial cells
- PMID: 20478312
- PMCID: PMC2917529
- DOI: 10.1016/j.yjmcc.2010.05.003
Sonic hedgehog induces angiogenesis via Rho kinase-dependent signaling in endothelial cells
Abstract
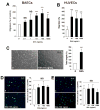
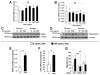
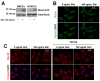
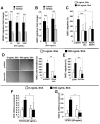
The morphogen Sonic hedgehog (Shh) promotes neovascularization in adults by inducing pro-angiogenic cytokine expression in fibroblasts; however, the direct effects of Shh on endothelial cell (EC) function during angiogenesis are unknown. Our findings indicate that Shh promotes capillary morphogenesis (tube length on Matrigel increased to 271+/-50% of the length in untreated cells, p=0.00003), induces EC migration (modified Boyden chamber assay, 191+/-35% of migration in untreated cells, p=0.00009), and increases EC expression of matrix metalloproteinase 9 (MMP-9) and osteopontin (OPN) mRNA (real-time RT-PCR), which are essential for Shh-induced angiogenesis both in vitro and in vivo. Shh activity in ECs is mediated by Rho, rather than through the "classic" Shh signaling pathway, which involves the Gli transcription factors. The Rho dependence of Shh-induced EC angiogenic activity was documented both in vitro, with dominant-negative RhoA and Rho kinase (ROCK) constructs, and in vivo, with the ROCK inhibitor Y27632 in the mouse corneal angiogenesis model. Finally, experiments performed in MMP-9- and OPN-knockout mice confirmed the roles of the ROCK downstream targets MMP-9 and OPN in Shh-induced angiogenesis. Collectively, our results identify a "nonclassical" pathway by which Shh directly modulates EC phenotype and angiogenic activity.
Copyright 2010 Elsevier Ltd. All rights reserved.
Figures

References
-
- Bitgood MJ, Shen L, McMahon AP. Sertoli cell signaling by Desert hedgehog regulates the male germline. Curr Biol. 1996;6:298–304. - PubMed
-
- Chiang C, Litingtung Y, Lee E, Young KE, Corden JL, Westphal H, et al. Cyclopia and defective axial patterning in mice lacking Sonic hedgehog gene function. Nature. 1996;383:407–13. - PubMed
-
- Johnson RL, Tabin CJ. Molecular models for vertebrate limb development. Cell. 1997;90:979–90. - PubMed
-
- Parmantier E, Lynn B, Lawson D, Turmaine M, Namini SS, Chakrabarti L, et al. Schwann cell-derived Desert hedgehog controls the development of peripheral nerve sheaths. Neuron. 1999;23:713–24. - PubMed
-
- Pepicelli CV, Lewis PM, McMahon AP. Sonic hedgehog regulates branching morphogenesis in the mammalian lung. Curr Biol. 1998;8:1083–6. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

