Characterization of the expression pattern of adrenergic receptors in rat taste buds
- PMID: 20478367
- PMCID: PMC2914163
- DOI: 10.1016/j.neuroscience.2010.05.021
Characterization of the expression pattern of adrenergic receptors in rat taste buds
Abstract
Taste buds signal the presence of chemical stimuli in the oral cavity to the central nervous system using both early transduction mechanisms, which allow single cells to be depolarized via receptor-mediated signaling pathways, and late transduction mechanisms, which involve extensive cell-to-cell communication among the cells in the bud. The latter mechanisms, which involve a large number of neurotransmitters and neuropeptides, are less well understood. Among neurotransmitters, multiple lines of evidence suggest that norepinephrine plays a yet unknown role in the taste bud. This study investigated the expression pattern of adrenergic receptors in the rat posterior taste bud. Expression of alpha1A, alpha1B, alpha1D, alpha2A, alpha2B, alpha2C, beta1, and the beta2 adrenoceptor subtypes was observed in taste buds using RT-PCR and immunocytochemical techniques. Taste buds also expressed the biosynthetic enzyme for norepinephrine, dopamine beta-hydroxylase (DbetaH), as well as the norepinephrine transporter. Further, expression of the epinephrine synthetic enzyme, phenylethanolamine N-methyltransferase (PNMT), was observed suggesting a possible role for this transmitter in the bud. Phenotyping adrenoceptor expression patterns with double labeling experiments to gustducin, synaptosomal-associated protein 25 (SNAP-25), and neural cell adhesion molecule (NCAM) suggests they are prominently expressed in subsets of cells known to express taste receptor molecules but segregated from cells known to have synapses with the afferent nerve fiber. Alpha and beta adrenoceptors co-express with one another in unique patterns as observed with immunocytochemistry and single cell reverse transcription polymerase chain reaction (RT-PCR). These data suggest that single cells express multiple adrenergic receptors and that adrenergic signaling may be particularly important in bitter, sweet, and umami taste qualities. In summary, adrenergic signaling in the taste bud occurs through complex pathways that include presynaptic and postsynaptic receptors and likely play modulatory roles in processing of gustatory information similar to other peripheral sensory systems such as the retina, cochlea, and olfactory bulb.
Copyright (c) 2010 IBRO. Published by Elsevier Ltd. All rights reserved.
Figures
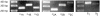
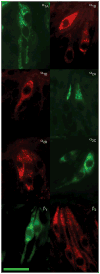

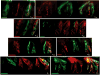
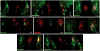

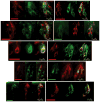

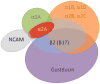
Similar articles
-
Co-expression patterns of the neuropeptides vasoactive intestinal peptide and cholecystokinin with the transduction molecules alpha-gustducin and T1R2 in rat taste receptor cells.Neuroscience. 2005;130(1):229-38. doi: 10.1016/j.neuroscience.2004.09.017. Neuroscience. 2005. PMID: 15561439
-
Expression of galanin and the galanin receptor in rat taste buds.Arch Histol Cytol. 2006 Dec;69(4):273-80. doi: 10.1679/aohc.69.273. Arch Histol Cytol. 2006. PMID: 17287581
-
Adrenergic signalling between rat taste receptor cells.J Physiol. 2002 Sep 1;543(Pt 2):601-14. doi: 10.1113/jphysiol.2002.020438. J Physiol. 2002. PMID: 12205193 Free PMC article.
-
Taste buds as peripheral chemosensory processors.Semin Cell Dev Biol. 2013 Jan;24(1):71-9. doi: 10.1016/j.semcdb.2012.12.002. Epub 2012 Dec 20. Semin Cell Dev Biol. 2013. PMID: 23261954 Free PMC article. Review.
-
The neuropeptides CCK and NPY and the changing view of cell-to-cell communication in the taste bud.Physiol Behav. 2009 Jul 14;97(5):581-91. doi: 10.1016/j.physbeh.2009.02.043. Epub 2009 Apr 5. Physiol Behav. 2009. PMID: 19332083 Review.
Cited by
-
β2-Adrenergic Receptor Utilizes Distinct Interaction Interfaces to Selectively form Heterooligomers with a Subset of Bitter Taste Receptors.Biochemistry. 2025 Aug 5;64(15):3219-3236. doi: 10.1021/acs.biochem.5c00208. Epub 2025 Jul 9. Biochemistry. 2025. PMID: 40631445 Free PMC article.
-
Chronic social defeat stress broadly inhibits gene expression in the peripheral taste system and alters taste responses in mice.Physiol Behav. 2024 Mar 1;275:114446. doi: 10.1016/j.physbeh.2023.114446. Epub 2023 Dec 20. Physiol Behav. 2024. PMID: 38128683 Free PMC article.
-
Bone marrow stromal and vascular smooth muscle cells have chemosensory capacity via bitter taste receptor expression.PLoS One. 2013;8(3):e58945. doi: 10.1371/journal.pone.0058945. Epub 2013 Mar 8. PLoS One. 2013. PMID: 23520545 Free PMC article.
-
Acetylcholine is released from taste cells, enhancing taste signalling.J Physiol. 2012 Jul 1;590(13):3009-17. doi: 10.1113/jphysiol.2012.232009. Epub 2012 May 8. J Physiol. 2012. PMID: 22570381 Free PMC article.
-
L-Amino Acids Elicit Diverse Response Patterns in Taste Sensory Cells: A Role for Multiple Receptors.PLoS One. 2015 Jun 25;10(6):e0130088. doi: 10.1371/journal.pone.0130088. eCollection 2015. PLoS One. 2015. PMID: 26110622 Free PMC article.
References
-
- Ando H, Tomida M, Inoue K, Asanuma N. Dopamine β-hydrolyase-like immunoreactive cells in the frog taste disc. Chemical Senses. 2007;32:825–832. - PubMed
-
- Bruss M, Porzgen P, Bryan-Lluka LJ, Bonisch H. The rat norepinephrine transporter: molecular cloning from PC12 cells and functional expression. Brain Res Mol Brain Res. 1997;52:257–262. - PubMed
-
- Bylund DB. Adrenergic receptors: Historical perspectives from the 20th century. In: Perez D, editor. The adrenergic receptors: in the 21st century. Ch1. Humana Press Inc; Totowa NJ: 2006.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

