Histone/protein deacetylase inhibitors increase suppressive functions of human FOXP3+ Tregs
- PMID: 20478744
- PMCID: PMC2917523
- DOI: 10.1016/j.clim.2010.04.018
Histone/protein deacetylase inhibitors increase suppressive functions of human FOXP3+ Tregs
Abstract
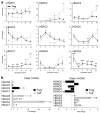
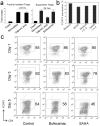
Histone/protein deacetylases (HDACs) decrease histone and protein acetylation, typically leading to suppression of gene transcription and modulation of various protein functions. We found significant differences in expression of HDAC before and after stimulation of human T regulatory (Treg) and T effector cells, suggesting the potential for future selective targeting of Tregs with HDAC inhibitors (HDACi). Use of various HDACi small molecules enhanced, by up to 4.5-fold (average 2-fold), the suppressive functions of both freshly isolated and expanded human Tregs, consistent with our previous murine data. HDACi use increased Treg expression of CTLA-4, a key negative regulator of immune response, and we found a direct and significant correlation between CTLA-4 expression and Treg suppression. Hence, HDACi compounds are promising pharmacologic tools to increase Treg suppressive functions, and this action may potentially be of use in patients with autoimmunity or post-transplantation.
Copyright 2010 Elsevier Inc. All rights reserved.
Figures







References
-
- Buckner JH, Ziegler SF. Functional analysis of FOXP3. Ann N Y Acad Sci. 2008;1143:151–69. - PubMed
-
- Shevach EM. Mechanisms of foxp3+ T regulatory cell-mediated suppression. Immunity. 2009;30:636–45. - PubMed
-
- Wing K, Sakaguchi S. Regulatory T cells exert checks and balances on self tolerance and autoimmunity. Nat Immunol. 2010;11:7–13. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

