Treatment of Resistant Depression in Adolescents (TORDIA): week 24 outcomes
- PMID: 20478877
- PMCID: PMC3257891
- DOI: 10.1176/appi.ajp.2010.09040552
Treatment of Resistant Depression in Adolescents (TORDIA): week 24 outcomes
Abstract
Objective: The purpose of this study was to report on the outcome of participants in the Treatment of Resistant Depression in Adolescents (TORDIA) trial after 24 weeks of treatment, including remission and relapse rates and predictors of treatment outcome.
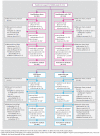
Method: Adolescents (ages 12-18 years) with selective serotonin reuptake inhibitor (SSRI)-resistant depression were randomly assigned to either a medication switch alone (alternate SSRI or venlafaxine) or a medication switch plus cognitive-behavioral therapy (CBT). At week 12, responders could continue in their assigned treatment arm and nonresponders received open treatment (medication and/or CBT) for 12 more weeks (24 weeks total). The primary outcomes were remission and relapse, defined by the Adolescent Longitudinal Interval Follow-Up Evaluation as rated by an independent evaluator.
Results: Of 334 adolescents enrolled in the study, 38.9% achieved remission by 24 weeks, and initial treatment assignment did not affect rates of remission. Likelihood of remission was much higher (61.6% versus 18.3%) and time to remission was much faster among those who had already demonstrated clinical response by week 12. Remission was also higher among those with lower baseline depression, hopelessness, and self-reported anxiety. At week 12, lower depression, hopelessness, anxiety, suicidal ideation, family conflict, and absence of comorbid dysthymia, anxiety, and drug/alcohol use and impairment also predicted remission. Of those who responded by week 12, 19.6% had a relapse of depression by week 24.
Conclusions: Continued treatment for depression among treatment-resistant adolescents results in remission in approximately one-third of patients, similar to adults. Eventual remission is evident within the first 6 weeks in many, suggesting that earlier intervention among nonresponders could be important.
Trial registration: ClinicalTrials.gov NCT00018902.
Figures


Comment in
-
Treatment of depressed adolescents.Am J Psychiatry. 2010 Jul;167(7):734-7. doi: 10.1176/appi.ajp.2010.10040566. Am J Psychiatry. 2010. PMID: 20595423 No abstract available.
References
-
- Bridge JA, Iyengar S, Salary CB, Barbe RP, Birmaher B, Pincus HA, Ren L, Brent DA. Clinical response and risk for reported suicidal ideation and suicide attempts in pediatric antidepressant treatment: a meta-analysis of randomized controlled trials. JAMA. 2007;297:1683–1696. - PubMed
-
- Brent D, Emslie G, Clarke G, Wagner KD, Asarnow JR, Keller M, Vitiello B, Ritz L, Iyengar S, Abebe K, Birmaher B, Ryan N, Kennard B, Hughes C, DeBar L, McCracken J, Strober M, Suddath R, Spirito A, Leonard H, Melhem N, Porta G, Onorato M, Zelazny J. Switching to another SSRI or to venlafaxine with or without cognitive behavioral therapy for adolescents with SSRI-resistant depression: the TORDIA randomized controlled trial. JAMA. 2008;299:901–913. - PMC - PubMed
-
- Asarnow JR, Emslie G, Clarke G, Wagner KD, Spirito A, Vitiello B, Iyengar S, Shamseddeen W, Ritz L, McCracken J, Strober M, Suddath R, Leonard H, Porta G, Keller M, Brent D. Treatment of selective serotonin reuptake inhibitor-resistant depression in adolescents: predictors and moderators of treatment response. J Am Acad Child Adolesc Psychiatry. 2009;48:330–339. - PMC - PubMed
-
- Brent DA, Emslie GJ, Clarke GN, Asarnow J, Spirito A, Ritz L, Vitiello B, Iyengar S, Birmaher B, Ryan ND, Zelazny J, Onorato M, Kennard B, Mayes TL, Debar LL, McCracken JT, Strober M, Suddath R, Leonard H, Porta G, Keller MB. Predictors of spontaneous and systematically assessed suicidal adverse events in the Treatment of SSRI-Resistant Depression in Adolescents (TORDIA) study. Am J Psychiatry. 2009;166:418–426. - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- U01 MH061835/MH/NIMH NIH HHS/United States
- U01 MH061958/MH/NIMH NIH HHS/United States
- U01 MH061864/MH/NIMH NIH HHS/United States
- MH-61856/MH/NIMH NIH HHS/United States
- P30 MH066371/MH/NIMH NIH HHS/United States
- MH-62014/MH/NIMH NIH HHS/United States
- U01 MH061869/MH/NIMH NIH HHS/United States
- MH-66371/MH/NIMH NIH HHS/United States
- MH-61958/MH/NIMH NIH HHS/United States
- U01 MH061856/MH/NIMH NIH HHS/United States
- MH-61835/MH/NIMH NIH HHS/United States
- U01 MH062014/MH/NIMH NIH HHS/United States
- MH-61864/MH/NIMH NIH HHS/United States
- MH-61869/MH/NIMH NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical

