Enteric glia modulate epithelial cell proliferation and differentiation through 15-deoxy-12,14-prostaglandin J2
- PMID: 20478974
- PMCID: PMC2916986
- DOI: 10.1113/jphysiol.2010.188409
Enteric glia modulate epithelial cell proliferation and differentiation through 15-deoxy-12,14-prostaglandin J2
Abstract
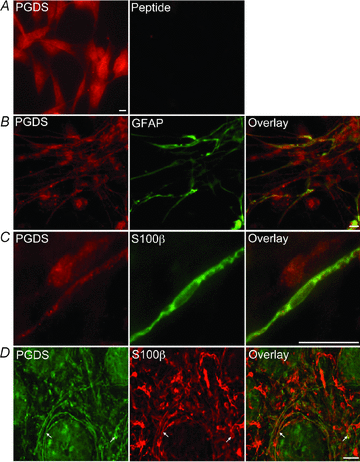
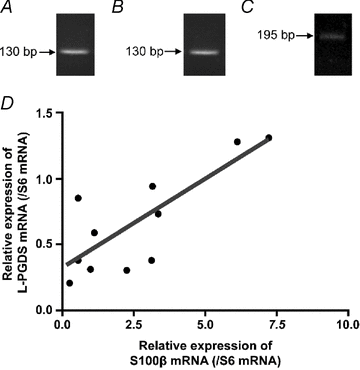
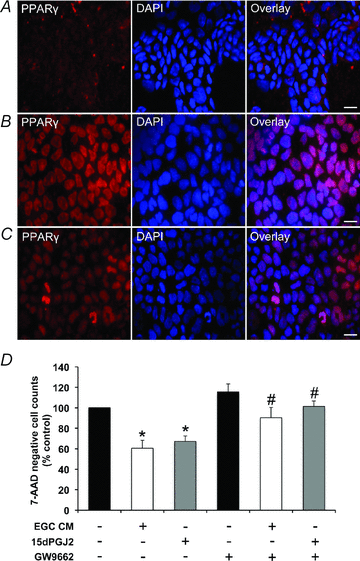
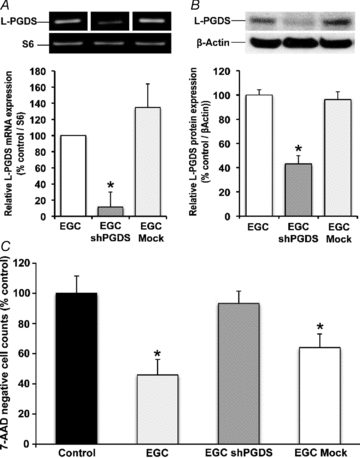
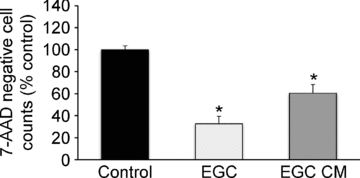
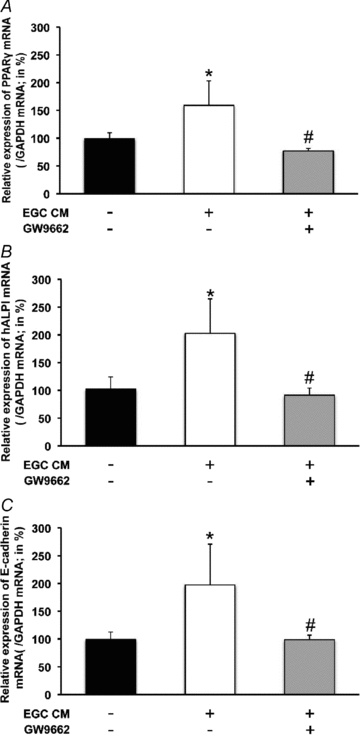
The enteric nervous system (ENS) and its major component, enteric glial cells (EGCs), have recently been identified as a major regulator of intestinal epithelial barrier functions. Indeed, EGCs inhibit intestinal epithelial cell (IEC) proliferation and increase barrier resistance and IEC adhesion via the release of EGC-derived soluble factors. Interestingly, EGC regulation of intestinal epithelial barrier functions is reminiscent of previously reported peroxisome proliferator-activated receptor gamma (PPARgamma)-dependent functional effects. In this context, the present study aimed at identifying whether EGC could synthesize and release the main PPARgamma ligand, 15-deoxy-(12,14)-prostaglandin J2 (15dPGJ2), and regulate IEC functions such as proliferation and differentiation via a PPARgamma dependent pathway. First, we demonstrated that the lipocalin but not the haematopoetic form for prostaglandin D synthase (PGDS), the enzyme responsible of 15dPGJ2 synthesis, was expressed in EGCs of the human submucosal plexus and of the subepithelium, as well as in rat primary culture of ENS and EGC lines. Next, 15dPGJ2 was identified in EGC supernatants of various EGC lines. 15dPGJ2 reproduced EGC inhibitory effects upon IEC proliferation, and inhibition of lipocalin PGDS expression by shRNA abrogated these effects. Furthermore, EGCs induced nuclear translocation of PPARgamma in IEC, and both EGC and 15dPGJ2 effects upon IEC proliferation were prevented by the PPARgamma antagonist GW9662. Finally, EGC induced differentiation-related gene expression in IEC through a PPARgamma-dependent pathway. Our results identified 15dPGJ2 as a novel glial-derived mediator involved in the control of IEC proliferation/differentiation through activation of PPARgamma. They also suggest that alterations of glial PGDS expression may modify intestinal epithelial barrier functions and be involved in the development of pathologies such as cancer or inflammatory bowel diseases.
Figures
References
-
- Beuckmann CT, Lazarus M, Gerashenko D, Mizoguchi A, Nomura S, Mohri I, Uesugi A, Kaneko T, Mizuno N, Hayaishi O, Urade Y. Cellular localization of lipocalin-type prostaglandin D synthase (beta-trace) in the central nervous sytem of the adult rat. J Comp Neurol. 2000;428:62–78. - PubMed
-
- Bull AW, Steffensen KR, Leers J, Rafter JJ. Activation of PPARγ in colon tumor cell lines by oxidized metabolites of linoleic acid, endogenous ligands for PPAR γ. Carcinogenesis. 2003;24:1717–1722. - PubMed
-
- Cabarrocas J, Savidge TC, Liblau RS. Role of enteric glial cells in inflammatory bowel disease. Glia. 2003;41:81–93. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

