Postnatal development of mouse heart: formation of energetic microdomains
- PMID: 20478976
- PMCID: PMC2915519
- DOI: 10.1113/jphysiol.2010.189670
Postnatal development of mouse heart: formation of energetic microdomains
Abstract
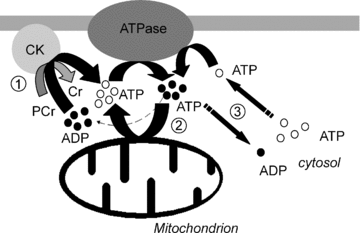
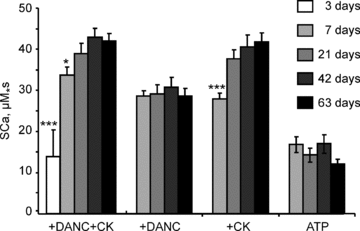
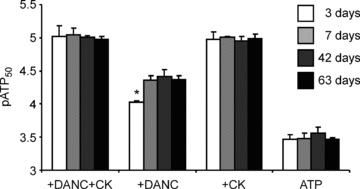
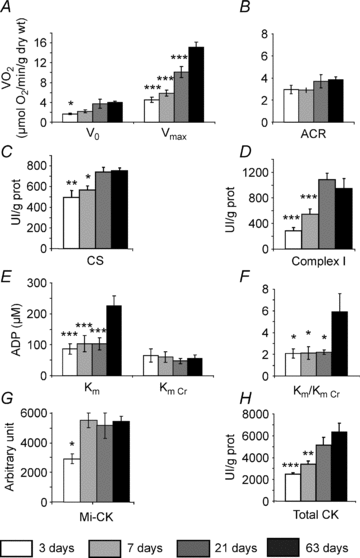
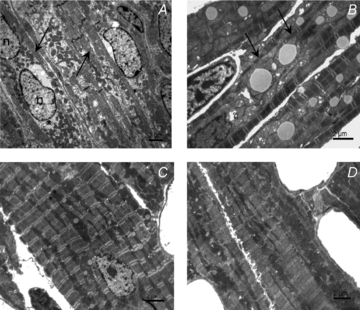
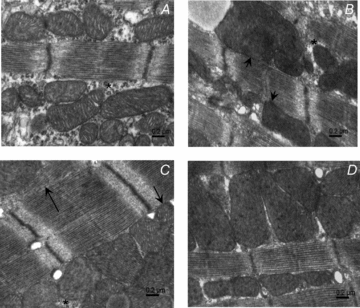
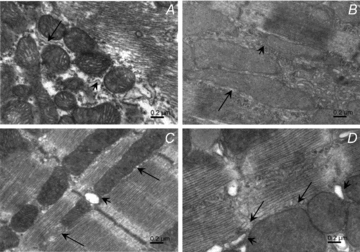
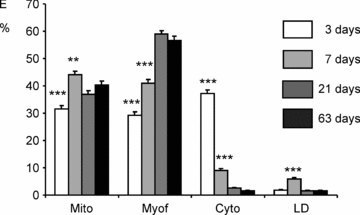
Cardiomyocyte contractile function requires tight control of the ATP/ADP ratio in the vicinity of the myosin-ATPase and sarcoplasmic reticulum ATPase (SERCA). In these cells, the main systems that provide energy are creatine kinase (CK), which catalyses phosphotransfer from phosphocreatine to ADP, and direct adenine nucleotide channelling (DANC) from mitochondria to ATPases. However, it is not known how and when these complex energetic systems are established during postnatal development. We therefore studied the maturation of the efficacy with which DANC and CK maintain ATP/ADP-dependent SR and myofibrillar function (SR Ca(2+) pumping and prevention of rigor tension), as well as the maturation of mitochondrial oxidative capacity. Experiments were performed on saponin-skinned fibres from left ventricles of 3-, 7-, 21-, 42- and 63-day-old mice. Cardiomyocyte and mitochondrial network morphology were characterized using electron microscopy. Our results show an early building-up of energetic microdomains in the developing mouse heart. CK efficacy for myosin-ATPase regulation was already maximal 3 days after birth, while for SERCA regulation it progressively increased until 21 days after birth. Seven days after birth, DANC for these two ATPases was as effective as in adult mice, despite a non-maximal mitochondrial respiration capacity. However, 3 days after birth, DANC between mitochondria and myosin-ATPase was not yet fully efficient. To prevent rigor tension in the presence of working mitochondria, the myosin-ATPase needed more intracellular MgATP in 3-day-old mice than in 7-day-old mice (pMgATP(50) 4.03 +/- 0.02 and 4.36 +/- 0.07, respectively, P < 0.05), whereas the intrinsic sensitivity of myofibrils to ATP (when mitochondria were inhibited) was similar at both ages. This may be due to the significant remodelling of the cytoarchitecture that occurs between these ages (cytosolic space reduction, formation of the mitochondrial network around the myofibrils). These results reveal a link between the maturation of intracellular energy pathways and cell architecture.
Figures

References
-
- Anmann T, Guzun R, Beraud N, Pelloux S, Kuznetsov AV, Kogerman L, Kaambre T, Sikk P, Paju K, Peet N, Seppet E, Ojeda C, Tourneur Y, Saks V. Different kinetics of the regulation of respiration in permeabilized cardiomyocytes and in HL-1 cardiac cells. Importance of cell structure/organization for respiration regulation. Biochim Biophys Acta. 2006;1757:1597–1606. - PubMed
-
- De Sousa E, Veksler V, Minajeva A, Kaasik A, Mateo P, Mayoux E, Hoerter J, Bigard X, Serrurier B, Ventura-Clapier R. Subcellular creatine kinase alterationsImplications in heart failure. Circ Res. 1999;85:68–76. - PubMed
-
- Estornell E, Fato R, Pallotti F, Lenaz G. Assay conditions for the mitochondrial NADH:coenzyme Q oxidoreductase. FEBS Lett. 1993;332:127–131. - PubMed
-
- Hew KW, Keller KA. Postnatal anatomical and functional development of the heart: a species comparison. Birth Defects Res B Dev Reprod Toxicol. 2003;68:309–320. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

