A new role for bicarbonate secretion in cervico-uterine mucus release
- PMID: 20478977
- PMCID: PMC2915510
- DOI: 10.1113/jphysiol.2010.187237
A new role for bicarbonate secretion in cervico-uterine mucus release
Abstract
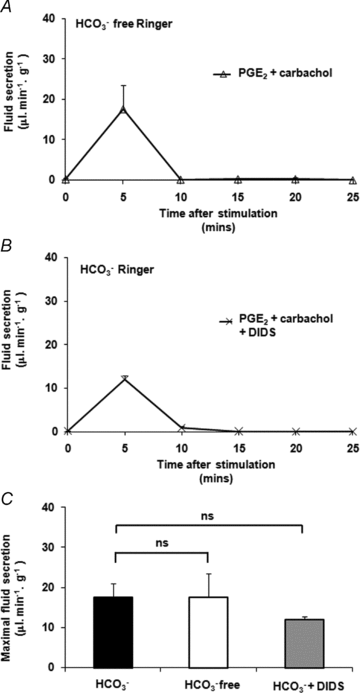
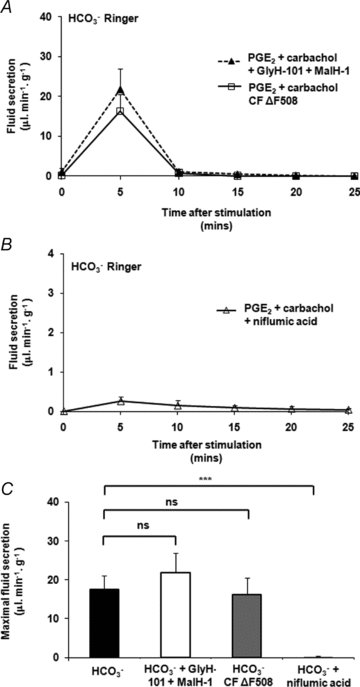
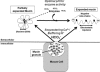
Cervical mucus thinning and release during the female reproductive cycle is thought to rely mainly on fluid secretion. However, we now find that mucus released from the murine reproductive tract critically depends upon concurrent bicarbonate (HCO(3)(-)) secretion. Prostaglandin E(2) (PGE(2))- and carbachol-stimulated mucus release was severely inhibited in the absence of serosal HCO(3)(-), HCO(3)(-) transport, or functional cystic fibrosis transmembrane conductance regulator (CFTR). In contrast to mucus release, PGE(2)- and carbachol-stimulated fluid secretion was not dependent on bicarbonate or on CFTR, but was completely blocked by niflumic acid. We found stimulated mucus release was severely impaired in the cystic fibrosis F508 reproductive tract, even though stimulated fluid secretion was preserved. Thus, CFTR mutations and/or poor bicarbonate secretion may be associated with reduced female fertility associated with abnormal mucus and specifically, may account for the increased viscosity and lack of cyclical changes in cervical mucus long noted in women with cystic fibrosis.
Figures



Comment in
-
Sparkling water--bicarbonate for cervix and cystic fibrosis.J Physiol. 2010 Aug 1;588(Pt 15):2685. doi: 10.1113/jphysiol.2010.194019. J Physiol. 2010. PMID: 20675814 Free PMC article. No abstract available.
References
-
- Andersch-Bjorkman Y, Thomsson KA, Holmen Larsson JM, Ekerhovd E, Hansson GC. Large scale identification of proteins, mucins, and their O-glycosylation in the endocervical mucus during the menstrual cycle. Mol Cell Proteomics. 2007;6:708–716. - PubMed
-
- Ansari AH, Gould KG, Ansari VM. Sodium bicarbonate douching for improvement of the postcoital test. Fertil Steril. 1980;33:608–612. - PubMed
-
- Argueso P, Spurr-Michaud S, Tisdale A, Gipson IK. Variation in the amount of T antigen and N-acetyllactosamine oligosaccharides in human cervical mucus secretions with the menstrual cycle. J Clin Endocrinol Metab. 2002;87:5641–5648. - PubMed
-
- Bhaskar KR, Turner BS, Grubman SA, Jefferson DM, LaMont JT. Dysregulation of proteoglycan production by intrahepatic biliary epithelial cells bearing defective (ΔF508) cystic fibrosis transmembrane conductance regulator. Hepatology. 1998;27:7–14. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

