Molecular determinants of the CaVbeta-induced plasma membrane targeting of the CaV1.2 channel
- PMID: 20478999
- PMCID: PMC2906277
- DOI: 10.1074/jbc.M110.111062
Molecular determinants of the CaVbeta-induced plasma membrane targeting of the CaV1.2 channel
Abstract
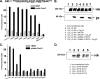
Ca(V)beta subunits modulate cell surface expression and voltage-dependent gating of high voltage-activated (HVA) Ca(V)1 and Ca(V)2 alpha1 subunits. High affinity Ca(V)beta binding onto the so-called alpha interaction domain of the I-II linker of the Ca(V)alpha1 subunit is required for Ca(V)beta modulation of HVA channel gating. It has been suggested, however, that Ca(V)beta-mediated plasma membrane targeting could be uncoupled from Ca(V)beta-mediated modulation of channel gating. In addition to Ca(V)beta, Ca(V)alpha2delta and calmodulin have been proposed to play important roles in HVA channel targeting. Indeed we show that co-expression of Ca(V)alpha2delta caused a 5-fold stimulation of the whole cell currents measured with Ca(V)1.2 and Ca(V)beta3. To gauge the synergetic role of auxiliary subunits in the steady-state plasma membrane expression of Ca(V)1.2, extracellularly tagged Ca(V)1.2 proteins were quantified using fluorescence-activated cell sorting analysis. Co-expression of Ca(V)1.2 with either Ca(V)alpha2delta, calmodulin wild type, or apocalmodulin (alone or in combination) failed to promote the detection of fluorescently labeled Ca(V)1.2 subunits. In contrast, co-expression with Ca(V)beta3 stimulated plasma membrane expression of Ca(V)1.2 by a 10-fold factor. Mutations within the alpha interaction domain of Ca(V)1.2 or within the nucleotide kinase domain of Ca(V)beta3 disrupted the Ca(V)beta3-induced plasma membrane targeting of Ca(V)1.2. Altogether, these data support a model where high affinity binding of Ca(V)beta to the I-II linker of Ca(V)alpha1 largely accounts for Ca(V)beta-induced plasma membrane targeting of Ca(V)1.2.
Figures

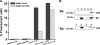


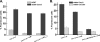
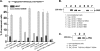
Similar articles
-
Functional properties of the CaV1.2 calcium channel activated by calmodulin in the absence of alpha2delta subunits.Channels (Austin). 2009 Jan-Feb;3(1):25-31. doi: 10.4161/chan.3.1.7498. Epub 2009 Jan 25. Channels (Austin). 2009. PMID: 19106618 Free PMC article.
-
Calmodulin-dependent gating of Ca(v)1.2 calcium channels in the absence of Ca(v)beta subunits.Proc Natl Acad Sci U S A. 2008 Jun 10;105(23):8154-9. doi: 10.1073/pnas.0711624105. Epub 2008 Jun 5. Proc Natl Acad Sci U S A. 2008. PMID: 18535142 Free PMC article.
-
A Polybasic Plasma Membrane Binding Motif in the I-II Linker Stabilizes Voltage-gated CaV1.2 Calcium Channel Function.J Biol Chem. 2015 Aug 21;290(34):21086-21100. doi: 10.1074/jbc.M115.645671. Epub 2015 Jun 22. J Biol Chem. 2015. PMID: 26100638 Free PMC article.
-
Voltage-dependent calcium channels.Gen Physiol Biophys. 2005 Jun;24 Suppl 1:1-78. Gen Physiol Biophys. 2005. PMID: 16096350 Review.
-
The ß subunit of voltage-gated Ca2+ channels.Physiol Rev. 2010 Oct;90(4):1461-506. doi: 10.1152/physrev.00057.2009. Physiol Rev. 2010. PMID: 20959621 Free PMC article. Review.
Cited by
-
Functional characterization of CaVα2δ mutations associated with sudden cardiac death.J Biol Chem. 2015 Jan 30;290(5):2854-69. doi: 10.1074/jbc.M114.597930. Epub 2014 Dec 19. J Biol Chem. 2015. PMID: 25527503 Free PMC article.
-
Identification of Glycosylation Sites Essential for Surface Expression of the CaVα2δ1 Subunit and Modulation of the Cardiac CaV1.2 Channel Activity.J Biol Chem. 2016 Feb 26;291(9):4826-43. doi: 10.1074/jbc.M115.692178. Epub 2016 Jan 7. J Biol Chem. 2016. PMID: 26742847 Free PMC article.
-
Mechanism of auxiliary β-subunit-mediated membrane targeting of L-type (Ca(V)1.2) channels.J Physiol. 2011 Sep 15;589(Pt 18):4437-55. doi: 10.1113/jphysiol.2011.214247. Epub 2011 Jul 11. J Physiol. 2011. PMID: 21746784 Free PMC article.
-
Proteolytic cleavage of the hydrophobic domain in the CaVα2δ1 subunit improves assembly and activity of cardiac CaV1.2 channels.J Biol Chem. 2017 Jun 30;292(26):11109-11124. doi: 10.1074/jbc.M117.784355. Epub 2017 May 11. J Biol Chem. 2017. PMID: 28495885 Free PMC article.
-
Trafficking of neuronal calcium channels.Neuronal Signal. 2017 Feb 20;1(1):NS20160003. doi: 10.1042/NS20160003. eCollection 2017 Feb. Neuronal Signal. 2017. PMID: 32714572 Free PMC article. Review.
References
-
- Snutch T. P., Reiner P. B. (1992) Curr. Opin. Neurobiol. 2, 247–253 - PubMed
-
- Zhang J. F., Randall A. D., Ellinor P. T., Horne W. A., Sather W. A., Tanabe T., Schwarz T. L., Tsien R. W. (1993) Neuropharmacology 32, 1075–1088 - PubMed
-
- Birnbaumer L., Campbell K. P., Catterall W. A., Harpold M. M., Hofmann F., Horne W. A., Mori Y., Schwartz A., Snutch T. P., Tanabe T. (1994) Neuron 13, 505–506 - PubMed
-
- Perez-Reyes E., Cribbs L. L., Daud A., Lacerda A. E., Barclay J., Williamson M. P., Fox M., Rees M., Lee J. H. (1998) Nature. 391, 896–900 - PubMed
-
- Cribbs L. L., Lee J. H., Yang J., Satin J., Zhang Y., Daud A., Barclay J., Williamson M. P., Fox M., Rees M., Perez-Reyes E. (1998) Circ. Res. 83, 103–109 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

