Leu85 in the beta1-beta2 linker of ASIC1 slows activation and decreases the apparent proton affinity by stabilizing a closed conformation
- PMID: 20479002
- PMCID: PMC2903392
- DOI: 10.1074/jbc.M110.134114
Leu85 in the beta1-beta2 linker of ASIC1 slows activation and decreases the apparent proton affinity by stabilizing a closed conformation
Abstract
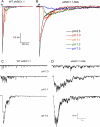
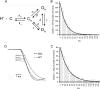
Acid-sensing ion channels (ASICs) are proton-activated channels expressed in neurons of the central and peripheral nervous systems where they modulate neuronal activity in response to external increases in proton concentration. The size of ASIC1 currents evoked by a given local acidification is determined by the number of channels in the plasma membrane and by the apparent proton affinities for activation and steady-state desensitization of the channel. Thus, the magnitude of the pH drop and the value of the baseline pH both are functionally important. Recent characterization of ASIC1s from an increasing number of species has made evident that proton affinities of these channels vary across vertebrates. We found that in species with high baseline plasma pH, e.g. frog, shark, and fish, ASIC1 has high proton affinity compared with the mammalian channel. The beta1-beta2 linker in the extracellular domain, specifically by the substitution M85L, determines the interspecies differences in proton affinities and also the time course of ASIC1 macroscopic currents. The mechanism underlying these observations is a delay in channel opening after application of protons, most likely by stabilizing a closed conformation that decreases the apparent affinity to protons and also slows the rise and decay phases of the current. Together, the results suggest evolutionary adaptation of ASIC1 to match the value of the species-specific plasma pH. At the molecular level, adaptation is achieved by substitutions of nonionizable residues rather than by modification of the channel proton sensor.
Figures

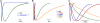
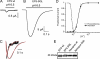
References
-
- Waldmann R., Champigny G., Bassilana F., Heurteaux C., Lazdunski M. (1997) Nature 386, 173–717 - PubMed
-
- Wemmie J. A., Chen J., Askwith C. C., Hruska-Hageman A. M., Price M. P., Nolan B. C., Yoder P. G., Lamani E., Hoshi T., Freeman J. H., Jr., Welsh M. J. (2002) Neuron 34, 463–477 - PubMed
-
- Jasti J., Furukawa H., Gonzales E. B., Gouaux E. (2007) Nature 449, 316–323 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

