A malignant hyperthermia-inducing mutation in RYR1 (R163C): alterations in Ca2+ entry, release, and retrograde signaling to the DHPR
- PMID: 20479110
- PMCID: PMC2888056
- DOI: 10.1085/jgp.200910328
A malignant hyperthermia-inducing mutation in RYR1 (R163C): alterations in Ca2+ entry, release, and retrograde signaling to the DHPR
Abstract
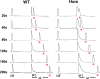
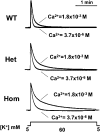
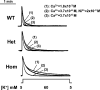
Bidirectional signaling between the sarcolemmal L-type Ca(2+) channel (1,4-dihydropyridine receptor [DHPR]) and the sarcoplasmic reticulum (SR) Ca(2+) release channel (type 1 ryanodine receptor [RYR1]) of skeletal muscle is essential for excitation-contraction coupling (ECC) and is a well-understood prototype of conformational coupling. Mutations in either channel alter coupling fidelity and with an added pharmacologic stimulus or stress can trigger malignant hyperthermia (MH). In this study, we measured the response of wild-type (WT), heterozygous (Het), or homozygous (Hom) RYR1-R163C knock-in mouse myotubes to maintained K(+) depolarization. The new findings are: (a) For all three genotypes, Ca(2+) transients decay during prolonged depolarization, and this decay is not a consequence of SR depletion or RYR1 inactivation. (b) The R163C mutation retards the decay rate with a rank order WT > Het > Hom. (c) The removal of external Ca(2+) or the addition of Ca(2+) entry blockers (nifedipine, SKF96365, and Ni(2+)) enhanced the rate of decay in all genotypes. (d) When Ca(2+) entry is blocked, the decay rates are slower for Hom and Het than WT, indicating that the rate of inactivation of ECC is affected by the R163C mutation and is genotype dependent (WT > Het > Hom). (e) Reduced ECC inactivation in Het and Hom myotubes was shown directly using two identical K(+) depolarizations separated by varying time intervals. These data suggest that conformational changes induced by the R163C MH mutation alter the retrograde signal that is sent from RYR1 to the DHPR, delaying the inactivation of the DHPR voltage sensor.
Figures


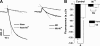
References
-
- Araya R., Liberona J.L., Cárdenas J.C., Riveros N., Estrada M., Powell J.A., Carrasco M.A., Jaimovich E. 2003. Dihydropyridine receptors as voltage sensors for a depolarization-evoked, IP3R-mediated, slow calcium signal in skeletal muscle cells. J. Gen. Physiol. 121:3–16 10.1085/jgp.20028671 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

