Superior antigen cross-presentation and XCR1 expression define human CD11c+CD141+ cells as homologues of mouse CD8+ dendritic cells
- PMID: 20479115
- PMCID: PMC2882837
- DOI: 10.1084/jem.20100348
Superior antigen cross-presentation and XCR1 expression define human CD11c+CD141+ cells as homologues of mouse CD8+ dendritic cells
Abstract
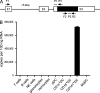
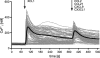
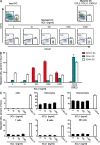
In recent years, human dendritic cells (DCs) could be subdivided into CD304+ plasmacytoid DCs (pDCs) and conventional DCs (cDCs), the latter encompassing the CD1c+, CD16+, and CD141+ DC subsets. To date, the low frequency of these DCs in human blood has essentially prevented functional studies defining their specific contribution to antigen presentation. We have established a protocol for an effective isolation of pDC and cDC subsets to high purity. Using this approach, we show that CD141+ DCs are the only cells in human blood that express the chemokine receptor XCR1 and respond to the specific ligand XCL1 by Ca2+ mobilization and potent chemotaxis. More importantly, we demonstrate that CD141+ DCs excel in cross-presentation of soluble or cell-associated antigen to CD8+ T cells when directly compared with CD1c+ DCs, CD16+ DCs, and pDCs from the same donors. Both in their functional XCR1 expression and their effective processing and presentation of exogenous antigen in the context of major histocompatibility complex class I, human CD141+ DCs correspond to mouse CD8+ DCs, a subset known for superior antigen cross-presentation in vivo. These data define CD141+ DCs as professional antigen cross-presenting DCs in the human.
Figures



References
-
- Caminschi I., Proietto A.I., Ahmet F., Kitsoulis S., Shin Teh J., Lo J.C., Rizzitelli A., Wu L., Vremec D., van Dommelen S.L., et al. 2008. The dendritic cell subtype-restricted C-type lectin Clec9A is a target for vaccine enhancement. Blood. 112:3264–3273 10.1182/blood-2008-05-155176 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

