Human CD141+ (BDCA-3)+ dendritic cells (DCs) represent a unique myeloid DC subset that cross-presents necrotic cell antigens
- PMID: 20479116
- PMCID: PMC2882828
- DOI: 10.1084/jem.20092140
Human CD141+ (BDCA-3)+ dendritic cells (DCs) represent a unique myeloid DC subset that cross-presents necrotic cell antigens
Abstract
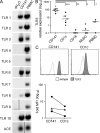
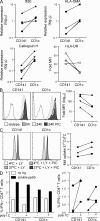
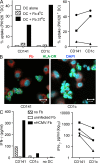
The characterization of human dendritic cell (DC) subsets is essential for the design of new vaccines. We report the first detailed functional analysis of the human CD141+ DC subset. CD141+ DCs are found in human lymph nodes, bone marrow, tonsil, and blood, and the latter proved to be the best source of highly purified cells for functional analysis. They are characterized by high expression of toll-like receptor 3, production of IL-12p70 and IFN-beta, and superior capacity to induce T helper 1 cell responses, when compared with the more commonly studied CD1c+ DC subset. Polyinosine-polycytidylic acid (poly I:C)-activated CD141+ DCs have a superior capacity to cross-present soluble protein antigen (Ag) to CD8+ cytotoxic T lymphocytes than poly I:C-activated CD1c+ DCs. Importantly, CD141+ DCs, but not CD1c+ DCs, were endowed with the capacity to cross-present viral Ag after their uptake of necrotic virus-infected cells. These findings establish the CD141+ DC subset as an important functionally distinct human DC subtype with characteristics similar to those of the mouse CD8alpha+ DC subset. The data demonstrate a role for CD141+ DCs in the induction of cytotoxic T lymphocyte responses and suggest that they may be the most relevant targets for vaccination against cancers, viruses, and other pathogens.
Figures






References
-
- Arrode G., Boccaccio C., Lulé J., Allart S., Moinard N., Abastado J.P., Alam A., Davrinche C. 2000. Incoming human cytomegalovirus pp65 (UL83) contained in apoptotic infected fibroblasts is cross-presented to CD8(+) T cells by dendritic cells. J. Virol. 74:10018–10024 10.1128/JVI.74.21.10018-10024.2000 - DOI - PMC - PubMed
-
- Bedoui S., Prato S., Mintern J., Gebhardt T., Zhan Y., Lew A.M., Heath W.R., Villadangos J.A., Segura E. 2009a. Characterization of an immediate splenic precursor of CD8+ dendritic cells capable of inducing antiviral T cell responses. J. Immunol. 182:4200–4207 10.4049/jimmunol.0802286 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

