Characterization of human DNGR-1+ BDCA3+ leukocytes as putative equivalents of mouse CD8alpha+ dendritic cells
- PMID: 20479117
- PMCID: PMC2882845
- DOI: 10.1084/jem.20092618
Characterization of human DNGR-1+ BDCA3+ leukocytes as putative equivalents of mouse CD8alpha+ dendritic cells
Abstract
In mouse, a subset of dendritic cells (DCs) known as CD8alpha+ DCs has emerged as an important player in the regulation of T cell responses and a promising target in vaccination strategies. However, translation into clinical protocols has been hampered by the failure to identify CD8alpha+ DCs in humans. Here, we characterize a population of human DCs that expresses DNGR-1 (CLEC9A) and high levels of BDCA3 and resembles mouse CD8alpha+ DCs in phenotype and function. We describe the presence of such cells in the spleens of humans and humanized mice and report on a protocol to generate them in vitro. Like mouse CD8alpha+ DCs, human DNGR-1+ BDCA3hi DCs express Necl2, CD207, BATF3, IRF8, and TLR3, but not CD11b, IRF4, TLR7, or (unlike CD8alpha+ DCs) TLR9. DNGR-1+ BDCA3hi DCs respond to poly I:C and agonists of TLR8, but not of TLR7, and produce interleukin (IL)-12 when given innate and T cell-derived signals. Notably, DNGR-1+ BDCA3+ DCs from in vitro cultures efficiently internalize material from dead cells and can cross-present exogenous antigens to CD8+ T cells upon treatment with poly I:C. The characterization of human DNGR-1+ BDCA3hi DCs and the ability to grow them in vitro opens the door for exploiting this subset in immunotherapy.
Figures


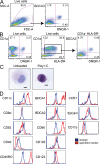
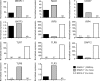
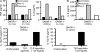
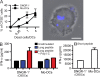
References
-
- Caminschi I., Proietto A.I., Ahmet F., Kitsoulis S., Shin Teh J., Lo J.C., Rizzitelli A., Wu L., Vremec D., van Dommelen S.L., et al. 2008. The dendritic cell subtype-restricted C-type lectin Clec9A is a target for vaccine enhancement. Blood. 112:3264–3273 10.1182/blood-2008-05-155176 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

