Molecular symbiosis of CHOP and C/EBP beta isoform LIP contributes to endoplasmic reticulum stress-induced apoptosis
- PMID: 20479126
- PMCID: PMC2897554
- DOI: 10.1128/MCB.01507-09
Molecular symbiosis of CHOP and C/EBP beta isoform LIP contributes to endoplasmic reticulum stress-induced apoptosis
Abstract
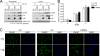
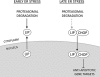
Induction of the transcription factor CHOP (CCAAT-binding homologous protein; GADD 153) is a critical cellular response for the transcriptional control of endoplasmic reticulum (ER) stress-induced apoptosis. Upon nuclear translocation, CHOP upregulates the transcription of proapoptotic factors and downregulates antiapoptotic genes. Transcriptional activation by CHOP involves heterodimerization with other members of the basic leucine zipper transcription factor (bZIP) family. We show that the bZIP protein C/EBP beta isoform LIP is required for nuclear translocation of CHOP during ER stress. In early ER stress, LIP undergoes proteasomal degradation in the cytoplasmic compartment. During later ER stress, LIP binds CHOP in both cytoplasmic and nuclear compartments and contributes to its nuclear import. By using CHOP-deficient cells and transfections of LIP-expressing vectors in C/EBP beta(-/-) mouse embryonic fibroblasts (MEFs), we show that the LIP-CHOP interaction has a stabilizing role for LIP. At the same time, CHOP uses LIP as a vehicle for nuclear import. LIP-expressing C/EBP beta(-/-) MEFs showed enhanced ER stress-induced apoptosis compared to C/EBP beta-null cells, a finding in agreement with the decreased levels of Bcl-2, a known transcriptional control target of CHOP. In view of the positive effect of CHOP-LIP interaction in mediating their proapoptotic functions, we propose this functional cooperativity as molecular symbiosis between proteins.
Figures




References
-
- Buck, M., V. Poli, T. Hunter, and M. Chojkier. 2001. C/EBPβ phosphorylation by RSK creates a functional XEXD caspase inhibitory box critical for cell survival. Mol. Cell 8:807-816. - PubMed
-
- Cao, Z., R. M. Umek, and S. L. McKnight. 1991. Regulated expression of three C/EBP isoforms during adipose conversion of 3T3-L1 cells. Genes Dev. 5:1538-1552. - PubMed
-
- Descombes, P., and U. Schibler. 1991. A liver-enriched transcriptional activator protein, LAP, and a transcriptional inhibitory protein, LIP, are translated from the same mRNA. Cell 67:569-579. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
