Cysteamine, the molecule used to treat cystinosis, potentiates the antimalarial efficacy of artemisinin
- PMID: 20479197
- PMCID: PMC2916301
- DOI: 10.1128/AAC.01719-09
Cysteamine, the molecule used to treat cystinosis, potentiates the antimalarial efficacy of artemisinin
Abstract
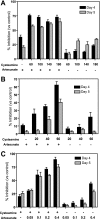
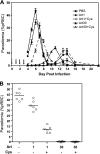
Malaria continues to be a major threat to global health. Artemisinin combination therapy (ACT) is the recommended treatment for clinical malaria; however, recent reports of parasite resistance to artemisinin in certain areas where malaria is endemic have stressed the need for developing more efficacious ACT. We report that cysteamine (Cys), the aminothiol used to treat nephropathic cystinosis in humans, strongly potentiates the efficacy of artemisinin against the Plasmodium parasite in vivo. Using a mouse model of infection with Plasmodium chabaudi AS, we observe that Cys dosing used to treat cystinosis in humans can strongly potentiate (by 3- to 4-fold) the antimalarial properties of the artemisinin derivatives artesunate and dihydroartemisinin. Addition of Cys to suboptimal doses of artemisinin delays the appearance of blood parasitemia, strongly reduces the extent of parasite replication, and significantly improves survival in a model of lethal P. chabaudi infection. Cys, the natural product of the enzyme pantetheinase, has a history of safe use for the clinical management of cystinosis. Our findings suggest that Cys could be included in novel ACTs to improve efficacy against Plasmodium parasite replication, including artemisinin-resistant isolates. Future work will include clinical evaluation of novel Cys-containing ACTs and elucidation of the mechanism underlying the potentiation effect of Cys.
Figures




Similar articles
-
Cysteamine broadly improves the anti-plasmodial activity of artemisinins against murine blood stage and cerebral malaria.Malar J. 2016 May 6;15(1):260. doi: 10.1186/s12936-016-1317-3. Malar J. 2016. PMID: 27150250 Free PMC article.
-
Genetic analysis in mice identifies cysteamine as a novel partner for artemisinin in the treatment of malaria.Mamm Genome. 2011 Aug;22(7-8):486-94. doi: 10.1007/s00335-011-9316-8. Epub 2011 Mar 25. Mamm Genome. 2011. PMID: 21437649 Review.
-
[Combined antimalarial therapy using artemisinin].Parassitologia. 2004 Jun;46(1-2):85-7. Parassitologia. 2004. PMID: 15305693 Review. Italian.
-
Cysteamine, the natural metabolite of pantetheinase, shows specific activity against Plasmodium.Exp Parasitol. 2010 Aug;125(4):315-24. doi: 10.1016/j.exppara.2010.02.009. Epub 2010 Feb 26. Exp Parasitol. 2010. PMID: 20219464 Free PMC article.
-
Plasmodium chabaudi: efficacy of artemisinin + curcumin combination treatment on a clone selected for artemisinin resistance in mice.Exp Parasitol. 2008 Jun;119(2):304-7. doi: 10.1016/j.exppara.2008.02.011. Epub 2008 Mar 7. Exp Parasitol. 2008. PMID: 18397786
Cited by
-
Cysteamine broadly improves the anti-plasmodial activity of artemisinins against murine blood stage and cerebral malaria.Malar J. 2016 May 6;15(1):260. doi: 10.1186/s12936-016-1317-3. Malar J. 2016. PMID: 27150250 Free PMC article.
-
Genetic control of susceptibility to infection with Plasmodium chabaudi chabaudi AS in inbred mouse strains.Genes Immun. 2012 Feb;13(2):155-63. doi: 10.1038/gene.2011.67. Epub 2011 Oct 6. Genes Immun. 2012. PMID: 21975430 Free PMC article.
-
Cysteamine inhibits lysosomal oxidation of low density lipoprotein in human macrophages and reduces atherosclerosis in mice.Atherosclerosis. 2019 Dec;291:9-18. doi: 10.1016/j.atherosclerosis.2019.09.019. Epub 2019 Sep 26. Atherosclerosis. 2019. PMID: 31629988 Free PMC article.
-
Harmine is a potent antimalarial targeting Hsp90 and synergizes with chloroquine and artemisinin.Antimicrob Agents Chemother. 2012 Aug;56(8):4207-13. doi: 10.1128/AAC.00328-12. Epub 2012 May 21. Antimicrob Agents Chemother. 2012. PMID: 22615284 Free PMC article.
-
Cysteamine, an Endogenous Aminothiol, and Cystamine, the Disulfide Product of Oxidation, Increase Pseudomonas aeruginosa Sensitivity to Reactive Oxygen and Nitrogen Species and Potentiate Therapeutic Antibiotics against Bacterial Infection.Infect Immun. 2018 May 22;86(6):e00947-17. doi: 10.1128/IAI.00947-17. Print 2018 Jun. Infect Immun. 2018. PMID: 29581193 Free PMC article.
References
-
- Adjuik, M., A. Babiker, P. Garner, P. Olliaro, W. Taylor, and N. White. 2004. Artesunate combinations for treatment of malaria: meta-analysis. Lancet 363:9-17. - PubMed
-
- Afonso, A., P. Hunt, S. Cheesman, A. C. Alves, C. V. Cunha, V. do Rosario, and P. Cravo. 2006. Malaria parasites can develop stable resistance to artemisinin but lack mutations in candidate genes atp6 (encoding the sarcoplasmic and endoplasmic reticulum Ca2+ ATPase), tctp, mdr1, and cg10. Antimicrob. Agents Chemother. 50:480-489. - PMC - PubMed
-
- Anikster, Y., V. Shotelersuk, and W. A. Gahl. 1999. CTNS mutations in patients with cystinosis. Hum. Mutat. 14:454-458. - PubMed
-
- Ayi, K., G. Min-Oo, L. Serghides, M. Crockett, M. Kirby-Allen, I. Quirt, P. Gros, and K. C. Kain. 2008. Pyruvate kinase deficiency and malaria. N. Engl. J. Med. 358:1805-1810. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

