Apolipoprotein E (APOE) genotype has dissociable effects on memory and attentional-executive network function in Alzheimer's disease
- PMID: 20479234
- PMCID: PMC2890481
- DOI: 10.1073/pnas.1001412107
Apolipoprotein E (APOE) genotype has dissociable effects on memory and attentional-executive network function in Alzheimer's disease
Abstract
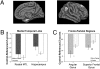
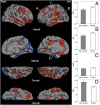
The epsilon4 allele of the apolipoprotein E (APOE) gene is the major genetic risk factor for Alzheimer's disease (AD), but limited work has suggested that APOE genotype may modulate disease phenotype. Carriers of the epsilon4 allele have been reported to have greater medial temporal lobe (MTL) pathology and poorer memory than noncarriers. Less attention has focused on whether there are domains of cognition and neuroanatomical regions more affected in noncarriers. Further, a major potential confound of prior in vivo studies is the possibility of different rates of clinical misdiagnosis for carriers vs. noncarriers. We compared phenotypic differences in cognition and topography of regional cortical atrophy of epsilon4 carriers (n = 67) vs. noncarriers (n = 24) with mild AD from the Alzheimer's Disease Neuroimaging Initiative, restricted to those with a cerebrospinal fluid (CSF) molecular profile consistent with AD. Between-group comparisons were made for psychometric tests and morphometric measures of cortical thickness and hippocampal volume. Carriers displayed significantly greater impairment on measures of memory retention, whereas noncarriers were more impaired on tests of working memory, executive control, and lexical access. Consistent with this cognitive dissociation, carriers exhibited greater MTL atrophy, whereas noncarriers had greater frontoparietal atrophy. Performance deficits in particular cognitive domains were associated with disproportionate regional brain atrophy within nodes of cortical networks thought to subserve these cognitive processes. These convergent cognitive and neuroanatomic findings in individuals with a CSF molecular profile consistent with AD support the hypothesis that APOE genotype modulates the clinical phenotype of AD through influence on specific large-scale brain networks.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- McKhann G, et al. Clinical diagnosis of Alzheimer’s disease: Report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34:939–944. - PubMed
-
- Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82:239–259. - PubMed
-
- Arnold SE, Hyman BT, Flory J, Damasio AR, Van Hoesen GW. The topographical and neuroanatomical distribution of neurofibrillary tangles and neuritic plaques in the cerebral cortex of patients with Alzheimer’s disease. Cereb Cortex. 1991;1:103–116. - PubMed
-
- Dubois B, et al. Research criteria for the diagnosis of Alzheimer’s disease: Revising the NINCDS-ADRDA criteria. Lancet Neurol. 2007;6:734–746. - PubMed
-
- Johnson JK, Head E, Kim R, Starr A, Cotman CW. Clinical and pathological evidence for a frontal variant of Alzheimer disease. Arch Neurol. 1999;56:1233–1239. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P50-AG005134/AG/NIA NIH HHS/United States
- P30AG010124/AG/NIA NIH HHS/United States
- P50 AG005134/AG/NIA NIH HHS/United States
- R21-AG29840/AG/NIA NIH HHS/United States
- R21 AG029840/AG/NIA NIH HHS/United States
- P30 AG019610/AG/NIA NIH HHS/United States
- R01 AG022374/AG/NIA NIH HHS/United States
- P30 AG010124/AG/NIA NIH HHS/United States
- R01 AG029411/AG/NIA NIH HHS/United States
- R01 AG027342/AG/NIA NIH HHS/United States
- U01 AG024904/AG/NIA NIH HHS/United States
- U19 AG010483/AG/NIA NIH HHS/United States
- R01-AG29411/AG/NIA NIH HHS/United States
- R01 AG012101/AG/NIA NIH HHS/United States
- K23-AG028018/AG/NIA NIH HHS/United States
- K23 AG028018/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

