Mucosal adjuvant activity of cholera toxin requires Th17 cells and protects against inhalation anthrax
- PMID: 20479237
- PMCID: PMC2890829
- DOI: 10.1073/pnas.1002348107
Mucosal adjuvant activity of cholera toxin requires Th17 cells and protects against inhalation anthrax
Abstract
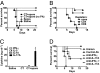
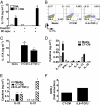
Cholera toxin (CT) elicits a mucosal immune response in mice when used as a vaccine adjuvant. The mechanisms by which CT exerts its adjuvant effects are incompletely understood. We show that protection against inhalation anthrax by an irradiated spore vaccine depends on CT-mediated induction of IL-17-producing CD4 Th17 cells. Furthermore, IL-17 is involved in the induction of serum and mucosal antibody responses by CT. Th17 cells induced by CT have a unique cytokine profile compared with those induced by IL-6 and TGF-beta, and their induction by CT requires cAMP-dependent secretion of IL-1beta and beta-calcitonin gene-related peptide by dendritic cells. These findings demonstrate that Th17 cells mediate mucosal adjuvant effects of CT and identify previously unexplored pathways involved in Th17 induction that could be targeted for development of unique mucosal adjuvants.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Northrup RS, Fauci AS. Adjuvant effect of cholera enterotoxin on the immune response of the mouse to sheep red blood cells. J Infect Dis. 1972;125:672–673. - PubMed
-
- Elson CO, Ealding W. Generalized systemic and mucosal immunity in mice after mucosal stimulation with cholera toxin. J Immunol. 1984;132:2736–2741. - PubMed
-
- Freytag LC, Clements JD. Mucosal adjuvants. Vaccine. 2005;23:1804–1813. - PubMed
-
- Vanden Broeck D, Horvath C, De Wolf MJ. Vibrio cholerae: Cholera toxin. Int J Biochem Cell Biol. 2007;39:1771–1775. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

