Role of CaMKIIdelta phosphorylation of the cardiac ryanodine receptor in the force frequency relationship and heart failure
- PMID: 20479242
- PMCID: PMC2890457
- DOI: 10.1073/pnas.1005843107
Role of CaMKIIdelta phosphorylation of the cardiac ryanodine receptor in the force frequency relationship and heart failure
Abstract
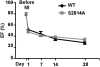
The force frequency relationship (FFR), first described by Bowditch 139 years ago as the observation that myocardial contractility increases proportionally with increasing heart rate, is an important mediator of enhanced cardiac output during exercise. Individuals with heart failure have defective positive FFR that impairs their cardiac function in response to stress, and the degree of positive FFR deficiency correlates with heart failure progression. We have identified a mechanism for FFR involving heart rate dependent phosphorylation of the major cardiac sarcoplasmic reticulum calcium release channel/ryanodine receptor (RyR2), at Ser2814, by calcium/calmodulin-dependent serine/threonine kinase-delta (CaMKIIdelta). Mice engineered with an RyR2-S2814A mutation have RyR2 channels that cannot be phosphorylated by CaMKIIdelta, and exhibit a blunted positive FFR. Ex vivo hearts from RyR2-S2814A mice also have blunted positive FFR, and cardiomyocytes isolated from the RyR2-S2814A mice exhibit impaired rate-dependent enhancement of cytosolic calcium levels and fractional shortening. The cardiac RyR2 macromolecular complexes isolated from murine and human failing hearts have reduced CaMKIIdelta levels. These data indicate that CaMKIIdelta phosphorylation of RyR2 plays an important role in mediating positive FFR in the heart, and that defective regulation of RyR2 by CaMKIIdelta-mediated phosphorylation is associated with the loss of positive FFR in failing hearts.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Comment in
-
How does CaMKIIdelta phosphorylation of the cardiac ryanodine receptor contribute to inotropy?Proc Natl Acad Sci U S A. 2010 Aug 3;107(31):E123; author reply E124. doi: 10.1073/pnas.1008809107. Epub 2010 Jul 21. Proc Natl Acad Sci U S A. 2010. PMID: 20660305 Free PMC article. No abstract available.
Similar articles
-
Calcium/Calmodulin Protein Kinase II-Dependent Ryanodine Receptor Phosphorylation Mediates Cardiac Contractile Dysfunction Associated With Sepsis.Crit Care Med. 2017 Apr;45(4):e399-e408. doi: 10.1097/CCM.0000000000002101. Crit Care Med. 2017. PMID: 27648519
-
Role of RyR2 phosphorylation at S2814 during heart failure progression.Circ Res. 2012 May 25;110(11):1474-83. doi: 10.1161/CIRCRESAHA.112.268094. Epub 2012 Apr 17. Circ Res. 2012. PMID: 22511749 Free PMC article.
-
Ablation of phospholamban rescues reperfusion arrhythmias but exacerbates myocardium infarction in hearts with Ca2+/calmodulin kinase II constitutive phosphorylation of ryanodine receptors.Cardiovasc Res. 2019 Mar 1;115(3):556-569. doi: 10.1093/cvr/cvy213. Cardiovasc Res. 2019. PMID: 30169578 Free PMC article.
-
Dysfunctional ryanodine receptors in the heart: new insights into complex cardiovascular diseases.J Mol Cell Cardiol. 2013 May;58:225-31. doi: 10.1016/j.yjmcc.2013.03.005. Epub 2013 Mar 16. J Mol Cell Cardiol. 2013. PMID: 23507255 Free PMC article. Review.
-
Cardiac ryanodine receptor phosphorylation by CaM Kinase II: keeping the balance right.Front Biosci (Landmark Ed). 2009 Jun 1;14(13):5134-56. doi: 10.2741/3591. Front Biosci (Landmark Ed). 2009. PMID: 19482609 Review.
Cited by
-
The neonatal but not the mature heart adapts to acute tachycardia by beneficial modification of the force-frequency relationship.Pediatr Cardiol. 2011 Jun;32(5):562-7. doi: 10.1007/s00246-011-9899-6. Epub 2011 Mar 11. Pediatr Cardiol. 2011. PMID: 21394656
-
Intracellular calcium release channels: an update.J Physiol. 2017 May 15;595(10):3041-3051. doi: 10.1113/JP272781. J Physiol. 2017. PMID: 28303572 Free PMC article. Review.
-
The ryanodine receptor in cardiac physiology and disease.Adv Pharmacol. 2010;59:1-30. doi: 10.1016/S1054-3589(10)59001-X. Adv Pharmacol. 2010. PMID: 20933197 Free PMC article. Review.
-
Neonatal mouse-derived engineered cardiac tissue: a novel model system for studying genetic heart disease.Circ Res. 2011 Jun 24;109(1):8-19. doi: 10.1161/CIRCRESAHA.111.242354. Epub 2011 May 12. Circ Res. 2011. PMID: 21566213 Free PMC article.
-
Aldosterone and cardiovascular disease: the heart of the matter.Trends Endocrinol Metab. 2013 Jan;24(1):21-30. doi: 10.1016/j.tem.2012.09.004. Epub 2012 Oct 3. Trends Endocrinol Metab. 2013. PMID: 23040074 Free PMC article. Review.
References
-
- Bowditch HP. Über die eigentümlichkeiten der reizbarkeit welche die muskelfasern des herzens zeigen (On the peculiarities of excitability which the fibers of cardiac muscle show) Ber Verh Saechs Akad Wiss. 1871;23:652–689.
-
- Allen DG, Blinks JR. Calcium transients in aequorin-injected frog cardiac muscle. Nature. 1978;273:509–513. - PubMed
-
- Braun AP, Schulman H. The multifunctional calcium/calmodulin-dependent protein kinase: From form to function. Annu Rev Physiol. 1995;57:417–445. - PubMed
-
- Maier LS, Bers DM. Role of Ca2+/calmodulin-dependent protein kinase (CaMK) in excitation-contraction coupling in the heart. Cardiovasc Res. 2007;73:631–640. - PubMed
-
- Miller SG, Kennedy MB. Regulation of brain type II Ca2+/calmodulin-dependent protein kinase by autophosphorylation: A Ca2+-triggered molecular switch. Cell. 1986;44:861–870. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

