Life-history connections to rates of aging in terrestrial vertebrates
- PMID: 20479246
- PMCID: PMC2890449
- DOI: 10.1073/pnas.1005862107
Life-history connections to rates of aging in terrestrial vertebrates
Abstract
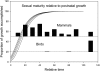
The actuarial senescence (i.e., the rate of increase in adult mortality with age) was related to body mass, development period, and age at sexual maturity across 124 taxonomic families of terrestrial vertebrates. Model selection based on Akaike's information criterion values adjusted for small size showed that the rate of aging decreases with increasing body mass, gestation period, age at maturity, and possession of flight. Among families of mammals, actuarial senescence was related to extrinsic mortality rate (standardized regression coefficient = 0.215), gestation period (-0.217), and age at maturity (-0.553). Although rate of aging in birds also was related to the embryo development period, birds grow several times more rapidly than mammals, and therefore, the connection between rate of early development and rate of aging is unclear. The strong vertebrate-wide relationship between rate of aging, or life span, and age at maturity can be explained by density-dependent feedback of adult survival rate on the recruitment of young individuals into the breeding population. Thus, age at maturity seems to reflect extrinsic mortality, which, in turn, influences selection on mechanisms that postpone physiological and actuarial senescence. Because rate of embryo development influences rate of aging independently of the age at maturity, in a statistical sense, the evolutionary diversification of development and aging seem to be connected in both birds and mammals; however, the linking mechanisms are not known.
Conflict of interest statement
The author declares no conflict of interest.
Figures

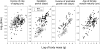



Similar articles
-
Glucocorticoids: mediators of vertebrate ontogenetic transitions.Gen Comp Endocrinol. 2008 May 1;156(3):441-53. doi: 10.1016/j.ygcen.2008.02.004. Epub 2008 Feb 16. Gen Comp Endocrinol. 2008. PMID: 18359027 Review.
-
Tyrannosaur ageing.Biol Lett. 2007 Apr 22;3(2):214-7. doi: 10.1098/rsbl.2006.0597. Biol Lett. 2007. PMID: 17284406 Free PMC article.
-
The evolution of senescence in fish.Mech Ageing Dev. 2002 Apr;123(7):773-89. doi: 10.1016/s0047-6374(01)00423-7. Mech Ageing Dev. 2002. PMID: 11869735 Review.
-
Insights from comparative analyses of aging in birds and mammals.Aging Cell. 2010 Apr;9(2):273-84. doi: 10.1111/j.1474-9726.2009.00542.x. Epub 2009 Dec 23. Aging Cell. 2010. PMID: 20041859 Free PMC article. Review.
-
Genome size and developmental parameters in the homeothermic vertebrates.Genome. 2002 Oct;45(5):833-8. doi: 10.1139/g02-050. Genome. 2002. PMID: 12416615
Cited by
-
The ecology of ageing in wild societies: linking age structure and social behaviour.Philos Trans R Soc Lond B Biol Sci. 2024 Dec 16;379(1916):20220464. doi: 10.1098/rstb.2022.0464. Epub 2024 Oct 28. Philos Trans R Soc Lond B Biol Sci. 2024. PMID: 39463244 Free PMC article. Review.
-
Age-structured Jolly-Seber model expands inference and improves parameter estimation from capture-recapture data.PLoS One. 2021 Jun 9;16(6):e0252748. doi: 10.1371/journal.pone.0252748. eCollection 2021. PLoS One. 2021. PMID: 34106979 Free PMC article.
-
Linking DNA damage and senescence to gestation period and lifespan in placental mammals.Front Cell Dev Biol. 2024 Sep 30;12:1480695. doi: 10.3389/fcell.2024.1480695. eCollection 2024. Front Cell Dev Biol. 2024. PMID: 39403126 Free PMC article.
-
Joint estimation of growth and survival from mark-recapture data to improve estimates of senescence in wild populations.Ecology. 2020 Jan;101(1):e02877. doi: 10.1002/ecy.2877. Epub 2019 Dec 26. Ecology. 2020. PMID: 31471965 Free PMC article.
-
Love and longevity: A Social Dependency Hypothesis.Compr Psychoneuroendocrinol. 2021 Sep 30;8:100088. doi: 10.1016/j.cpnec.2021.100088. eCollection 2021 Nov. Compr Psychoneuroendocrinol. 2021. PMID: 35757670 Free PMC article. Review.
References
-
- Finch CE, Ruvkun G. The genetics of aging. Annu Rev Genomics Hum Genet. 2001;2:435–462. - PubMed
-
- Partridge L, Barton NH. Evolution of aging: Testing the theory using Drosophila. Genetica. 1993;91:89–98. - PubMed
-
- Rose MR. Evolutionary Biology of Aging. New York: Oxford University Press; 1991.
-
- Barja G. Aging in vertebrates, and the effect of caloric restriction: A mitochondrial free radical production-DNA damage mechanism? Biol Rev Camb Philos Soc. 2004;79:235–251. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

