Integrated molecular genetic profiling of pediatric high-grade gliomas reveals key differences with the adult disease
- PMID: 20479398
- PMCID: PMC2903336
- DOI: 10.1200/JCO.2009.26.7252
Integrated molecular genetic profiling of pediatric high-grade gliomas reveals key differences with the adult disease
Abstract
Purpose: To define copy number alterations and gene expression signatures underlying pediatric high-grade glioma (HGG).
Patients and methods: We conducted a high-resolution analysis of genomic imbalances in 78 de novo pediatric HGGs, including seven diffuse intrinsic pontine gliomas, and 10 HGGs arising in children who received cranial irradiation for a previous cancer using single nucleotide polymorphism microarray analysis. Gene expression was analyzed with gene expression microarrays for 53 tumors. Results were compared with publicly available data from adult tumors.
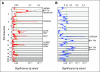
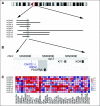
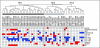
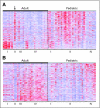
Results: Significant differences in copy number alterations distinguish childhood and adult glioblastoma. PDGFRA was the predominant target of focal amplification in childhood HGG, including diffuse intrinsic pontine gliomas, and gene expression analyses supported an important role for deregulated PDGFRalpha signaling in pediatric HGG. No IDH1 hotspot mutations were found in pediatric tumors, highlighting molecular differences with adult secondary glioblastoma. Pediatric and adult glioblastomas were clearly distinguished by frequent gain of chromosome 1q (30% v 9%, respectively) and lower frequency of chromosome 7 gain (13% v 74%, respectively) and 10q loss (35% v 80%, respectively). PDGFRA amplification and 1q gain occurred at significantly higher frequency in irradiation-induced tumors, suggesting that these are initiating events in childhood gliomagenesis. A subset of pediatric HGGs showed minimal copy number changes.
Conclusion: Integrated molecular profiling showed substantial differences in the molecular features underlying pediatric and adult HGG, indicating that findings in adult tumors cannot be simply extrapolated to younger patients. PDGFRalpha may be a useful target for pediatric HGG, including diffuse pontine gliomas.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Figures

References
-
- Broniscer A, Gajjar A. Supratentorial high-grade astrocytoma and diffuse brainstem glioma: Two challenges for the pediatric oncologist. Oncologist. 2004;9:197–206. - PubMed
-
- Broniscer A, Baker SJ, West AN, et al. Clinical and molecular characteristics of malignant transformation of low-grade glioma in children. J Clin Oncol. 2007;25:682–689. - PubMed
-
- Furnari FB, Fenton T, Bachoo RM, et al. Malignant astrocytic glioma: Genetics, biology, and paths to treatment. Genes Dev. 2007;21:2683–2710. - PubMed
-
- Bredel M, Bredel C, Juric D, et al. High-resolution genome-wide mapping of genetic alterations in human glial brain tumors. Cancer Res. 2005;65:4088–4096. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

