Akt phosphorylation at Ser473 predicts benefit of paclitaxel chemotherapy in node-positive breast cancer
- PMID: 20479407
- PMCID: PMC2903333
- DOI: 10.1200/JCO.2009.26.1602
Akt phosphorylation at Ser473 predicts benefit of paclitaxel chemotherapy in node-positive breast cancer
Abstract
Purpose: We tested the hypothesis that Akt-Ser473 phosphorylation (pAkt) predicts benefit from the sequential addition of paclitaxel to adjuvant doxorubicin plus cyclophosphamide (AC) chemotherapy in patients with node-positive breast cancer participating in the National Surgical Adjuvant Breast and Bowel Project (NSABP) B-28 trial.
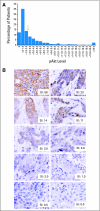
Patients and methods: Primary tumors from the NSABP B-28 trial tissue microarray were available from 1,581 of 3,060 patients who were randomly assigned to receive either four cycles of AC alone or followed by four cycles of paclitaxel. Immunohistochemistry and quantitative analysis of pAkt were performed at the National Cancer Institute blinded to clinical outcome. Association between pAkt and clinical outcome was assessed using multivariate Cox modeling adjusting for age, tumor size, number of positive nodes, tumor grade, estrogen receptor status, and human epidermal growth factor receptor 2 status.
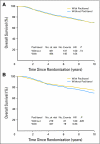
Results: With a median follow-up of 9.1 years, there were no differences in disease-free survival (adjusted hazard ratio [HR], 1.02; P = .81) or overall survival (HR, 0.97; P = .80) with and without receiving paclitaxel among 975 patients with pAkt-negative tumors. In 606 patients with pAkt-positive tumors, the sequential addition of paclitaxel resulted in a 26% improvement in disease-free survival (HR, 0.74; P = .02) or a 20% improvement in overall survival (HR, 0.80; P = .17).
Conclusion: pAkt significantly predicts disease-free benefit from the sequential addition of paclitaxel to AC chemotherapy in patients with node-positive breast cancer. Patients with pAkt-negative breast tumors do not appear to benefit from the addition of paclitaxel.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Figures





Similar articles
-
Paclitaxel after doxorubicin plus cyclophosphamide as adjuvant chemotherapy for node-positive breast cancer: results from NSABP B-28.J Clin Oncol. 2005 Jun 1;23(16):3686-96. doi: 10.1200/JCO.2005.10.517. Epub 2005 May 16. J Clin Oncol. 2005. PMID: 15897552 Clinical Trial.
-
HER2 and response to paclitaxel in node-positive breast cancer.N Engl J Med. 2007 Oct 11;357(15):1496-506. doi: 10.1056/NEJMoa071167. N Engl J Med. 2007. PMID: 17928597 Clinical Trial.
-
21-Gene Recurrence Score for prognosis and prediction of taxane benefit after adjuvant chemotherapy plus endocrine therapy: results from NSABP B-28/NRG Oncology.Breast Cancer Res Treat. 2018 Feb;168(1):69-77. doi: 10.1007/s10549-017-4550-8. Epub 2017 Nov 11. Breast Cancer Res Treat. 2018. PMID: 29128898 Free PMC article. Clinical Trial.
-
Classical cyclophosphamide, methotrexate, and fluorouracil chemotherapy is more effective in triple-negative, node-negative breast cancer: results from two randomized trials of adjuvant chemoendocrine therapy for node-negative breast cancer.J Clin Oncol. 2010 Jun 20;28(18):2966-73. doi: 10.1200/JCO.2009.25.9549. Epub 2010 May 10. J Clin Oncol. 2010. PMID: 20458051 Free PMC article. Clinical Trial.
-
Adjuvant therapy approaches to breast cancer: should taxanes be incorporated?Curr Oncol Rep. 2003 Jan;5(1):66-71. doi: 10.1007/s11912-003-0089-4. Curr Oncol Rep. 2003. PMID: 12493153 Review.
Cited by
-
Extreme loss of immunoreactive p-Akt and p-Erk1/2 during routine fixation of primary breast cancer.Breast Cancer Res. 2010;12(5):R76. doi: 10.1186/bcr2719. Epub 2010 Sep 28. Breast Cancer Res. 2010. PMID: 20920193 Free PMC article.
-
TGF-β causes Docetaxel resistance in Prostate Cancer via the induction of Bcl-2 by acetylated KLF5 and Protein Stabilization.Theranostics. 2020 Jun 18;10(17):7656-7670. doi: 10.7150/thno.44567. eCollection 2020. Theranostics. 2020. PMID: 32685011 Free PMC article.
-
Taxane resistance in castration-resistant prostate cancer: mechanisms and therapeutic strategies.Acta Pharm Sin B. 2018 Jul;8(4):518-529. doi: 10.1016/j.apsb.2018.04.007. Epub 2018 Apr 30. Acta Pharm Sin B. 2018. PMID: 30109177 Free PMC article. Review.
-
Differential involvement of the microtubule cytoskeleton in insulin receptor substrate 1 (IRS-1) and IRS-2 signaling to AKT determines the response to microtubule disruption in breast carcinoma cells.J Biol Chem. 2017 May 12;292(19):7806-7816. doi: 10.1074/jbc.M117.785832. Epub 2017 Mar 20. J Biol Chem. 2017. PMID: 28320862 Free PMC article.
-
The Akt signaling pathway: an emerging therapeutic target in malignant melanoma.Cancer Biol Ther. 2011 Dec 15;12(12):1032-49. doi: 10.4161/cbt.12.12.18442. Epub 2011 Dec 15. Cancer Biol Ther. 2011. PMID: 22157148 Free PMC article. Review.
References
-
- Early Breast Cancer Trialists' Collaborative Group: Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: An overview of the randomised trials. Lancet. 2005;365:1687–1717. - PubMed
-
- De Laurentiis M, Cancello G, D'Agostino D, et al. Taxane-based combinations as adjuvant chemotherapy of early breast cancer: A meta-analysis of randomized trials. J Clin Oncol. 2008;26:44–53. - PubMed
-
- Montero A, Fossella F, Hortobagyi G, et al. Docetaxel for treatment of solid tumours: A systematic review of clinical data. Lancet Oncol. 2005;6:229–239. - PubMed
-
- Vasey PA, Jayson GC, Gordon A, et al. Phase III randomized trial of docetaxel-carboplatin versus paclitaxel-carboplatin as first-line chemotherapy for ovarian carcinoma. J Natl Cancer Inst. 2004;96:1682–1691. - PubMed
-
- Mamounas EP, Bryant J, Lembersky B, et al. Paclitaxel after doxorubicin plus cyclophosphamide as adjuvant chemotherapy for node-positive breast cancer: Results from NSABP B-28. J Clin Oncol. 2005;23:3686–3696. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

