Global chromatin architecture reflects pluripotency and lineage commitment in the early mouse embryo
- PMID: 20479880
- PMCID: PMC2866533
- DOI: 10.1371/journal.pone.0010531
Global chromatin architecture reflects pluripotency and lineage commitment in the early mouse embryo
Abstract
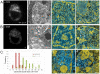
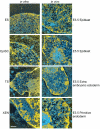
An open chromatin architecture devoid of compact chromatin is thought to be associated with pluripotency in embryonic stem cells. Establishing this distinct epigenetic state may also be required for somatic cell reprogramming. However, there has been little direct examination of global structural domains of chromatin during the founding and loss of pluripotency that occurs in preimplantation mouse development. Here, we used electron spectroscopic imaging to examine large-scale chromatin structural changes during the transition from one-cell to early postimplantation stage embryos. In one-cell embryos chromatin was extensively dispersed with no noticeable accumulation at the nuclear envelope. Major changes were observed from one-cell to two-cell stage embryos, where chromatin became confined to discrete blocks of compaction and with an increased concentration at the nuclear envelope. In eight-cell embryos and pluripotent epiblast cells, chromatin was primarily distributed as an extended meshwork of uncompacted fibres and was indistinguishable from chromatin organization in embryonic stem cells. In contrast, lineage-committed trophectoderm and primitive endoderm cells, and the stem cell lines derived from these tissues, displayed higher levels of chromatin compaction, suggesting an association between developmental potential and chromatin organisation. We examined this association in vivo and found that deletion of Oct4, a factor required for pluripotency, caused the formation of large blocks of compact chromatin in putative epiblast cells. Together, these studies show that an open chromatin architecture is established in the embryonic lineages during development and is sufficient to distinguish pluripotent cells from tissue-restricted progenitor cells.
Conflict of interest statement
Figures






References
-
- Rossant J, Tam PP. Blastocyst lineage formation, early embryonic asymmetries and axis patterning in the mouse. Development. 2009;136:701–713. - PubMed
-
- Smith AG. Embryo-derived stem cells: of mice and men. Annu Rev Cell Dev Biol. 2001;17:435–462. - PubMed
-
- Kornberg RD, Lorch Y. Irresistible force meets immovable object: transcription and the nucleosome. Cell. 1991;67:833–836. - PubMed
-
- Kornberg RD, Lorch Y. Chromatin structure and transcription. Annu Rev Cell Biol. 1992;8:563–587. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

