Application of the principles of systems biology and Wiener's cybernetics for analysis of regulation of energy fluxes in muscle cells in vivo
- PMID: 20479996
- PMCID: PMC2869234
- DOI: 10.3390/ijms11030982
Application of the principles of systems biology and Wiener's cybernetics for analysis of regulation of energy fluxes in muscle cells in vivo
Abstract
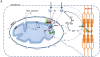
The mechanisms of regulation of respiration and energy fluxes in the cells are analyzed based on the concepts of systems biology, non-equilibrium steady state kinetics and applications of Wiener's cybernetic principles of feedback regulation. Under physiological conditions cardiac function is governed by the Frank-Starling law and the main metabolic characteristic of cardiac muscle cells is metabolic homeostasis, when both workload and respiration rate can be changed manifold at constant intracellular level of phosphocreatine and ATP in the cells. This is not observed in skeletal muscles. Controversies in theoretical explanations of these observations are analyzed. Experimental studies of permeabilized fibers from human skeletal muscle vastus lateralis and adult rat cardiomyocytes showed that the respiration rate is always an apparent hyperbolic but not a sigmoid function of ADP concentration. It is our conclusion that realistic explanations of regulation of energy fluxes in muscle cells require systemic approaches including application of the feedback theory of Wiener's cybernetics in combination with detailed experimental research. Such an analysis reveals the importance of limited permeability of mitochondrial outer membrane for ADP due to interactions of mitochondria with cytoskeleton resulting in quasi-linear dependence of respiration rate on amplitude of cyclic changes in cytoplasmic ADP concentrations. The system of compartmentalized creatine kinase (CK) isoenzymes functionally coupled to ANT and ATPases, and mitochondrial-cytoskeletal interactions separate energy fluxes (mass and energy transfer) from signalling (information transfer) within dissipative metabolic structures - intracellular energetic units (ICEU). Due to the non-equilibrium state of CK reactions, intracellular ATP utilization and mitochondrial ATP regeneration are interconnected by the PCr flux from mitochondria. The feedback regulation of respiration occurring via cyclic fluctuations of cytosolic ADP, Pi and Cr/PCr ensures metabolic stability necessary for normal function of cardiac cells.
Keywords: cytoskeleton; metabolic homeostasis; mitochondria; muscle cells; phosphotransfer networks; regulation; respiration; systems biology.
Figures
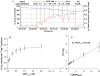



 ) – in the presence of both trapping system for free ADP and 20 mM creatine. Reproduced from [32] with permission.
) – in the presence of both trapping system for free ADP and 20 mM creatine. Reproduced from [32] with permission.






References
-
- Noble D. Modeling the heart--from genes to cells to the whole organ. Science. 2002;295:1678–1682. - PubMed
-
- Alberghina L, Westerhoff HV. Systems Biology: Definitions and Perspectives (Topics in Current Genetics) Springer Verlag; Berlin, Heidelberg, Germany: 2005.
-
- Noble D. The Music of Life: Biology Beyond the Genome. Oxford University Press; Oxford, UK: 2006.
-
- Westerhoff HV, Kolodkin A, Conradie R, Wilkinson SJ, Bruggeman FJ, Krab K, van Schuppen JH, Hardin H, Bakker BM, Mone MJ, Rybakova KN, Eijken M, van Leeuwen HJ, Snoep JL. Systems biology towards life in silico: Mathematics of the control of living cells. J. Math. Biol. 2009;58:7–34. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

