A review on progress in QSPR studies for surfactants
- PMID: 20479997
- PMCID: PMC2868353
- DOI: 10.3390/ijms11031020
A review on progress in QSPR studies for surfactants
Abstract
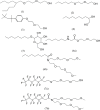
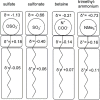
This paper presents a review on recent progress in quantitative structure-property relationship (QSPR) studies of surfactants and applications of various molecular descriptors. QSPR studies on critical micelle concentration (cmc) and surface tension (gamma) of surfactants are introduced. Studies on charge distribution in ionic surfactants by quantum chemical calculations and its effects on the structures and properties of the colloids of surfactants are also reviewed. The trends of QSPR studies on cloud point (for nonionic surfactants), biodegradation potential and some other properties of surfactants are evaluated.
Keywords: QSPR; biodegradation; cloud point; cmc; surface tension; surfactants.
Figures


Similar articles
-
Prediction of critical micelle concentration of nonionic surfactants by a quantitative structure - property relationship.Comb Chem High Throughput Screen. 2010 Jan;13(1):39-44. doi: 10.2174/138620710790218195. Comb Chem High Throughput Screen. 2010. PMID: 20201824
-
QSPR for the prediction of critical micelle concentration of different classes of surfactants using machine learning algorithms.J Mol Graph Model. 2024 Jun;129:108757. doi: 10.1016/j.jmgm.2024.108757. Epub 2024 Mar 11. J Mol Graph Model. 2024. PMID: 38503002
-
Combination of genetic algorithm and partial least squares for cloud point prediction of nonionic surfactants from molecular structures.Ann Chim. 2007 Jan-Feb;97(1-2):69-83. doi: 10.1002/adic.200690087. Ann Chim. 2007. PMID: 17822265
-
General theory for multiple input-output perturbations in complex molecular systems. 1. Linear QSPR electronegativity models in physical, organic, and medicinal chemistry.Curr Top Med Chem. 2013;13(14):1713-41. doi: 10.2174/1568026611313140011. Curr Top Med Chem. 2013. PMID: 23889050 Review.
-
Biomimetic amphiphiles: properties and potential use.Adv Exp Med Biol. 2010;672:102-20. doi: 10.1007/978-1-4419-5979-9_8. Adv Exp Med Biol. 2010. PMID: 20545277 Review.
Cited by
-
Quantitative Structure-Toxicity Relationship in Bioactive Molecules from a Conceptual DFT Perspective.Pharmaceuticals (Basel). 2022 Nov 10;15(11):1383. doi: 10.3390/ph15111383. Pharmaceuticals (Basel). 2022. PMID: 36355555 Free PMC article. Review.
-
Nanoscale zero-valent metals: a review of synthesis, characterization, and applications to environmental remediation.Environ Sci Pollut Res Int. 2016 Sep;23(18):17880-900. doi: 10.1007/s11356-016-6626-0. Epub 2016 Apr 20. Environ Sci Pollut Res Int. 2016. PMID: 27094266 Review.
-
Structure-based modeling of critical micelle concentration (CMC) of anionic surfactants in brine using intelligent methods.Sci Rep. 2023 Aug 17;13(1):13361. doi: 10.1038/s41598-023-40466-1. Sci Rep. 2023. PMID: 37591920 Free PMC article.
-
Optimization of Toxicity, Biodegradability, and Skin Irritation in Formulations Containing Mixtures of Anionic and Nonionic Surfactants Combined with Silica Nanoparticles.Toxics. 2025 Jan 8;13(1):43. doi: 10.3390/toxics13010043. Toxics. 2025. PMID: 39853041 Free PMC article.
-
Computational Profiling of Monoterpenoid Phytochemicals: Insights for Medicinal Chemistry and Drug Design Strategies.Int J Mol Sci. 2025 Aug 8;26(16):7671. doi: 10.3390/ijms26167671. Int J Mol Sci. 2025. PMID: 40868994 Free PMC article.
References
-
- Li GZ, Sui H, Zhu WZ. Progress of surfactant study [in Chinese] China Surfact. Deterg. Cosm. 1999;1:24–26.
-
- William AE, the Committee on Fetus and Newborn Surfactant-replacement therapy for respiratory distress in the preterm and term neonate. Pediatrics. 2008;121:419–432. - PubMed
-
- Riviere JE, Baynes RE, Xia XR. Membrane-coated fiber array approach for predicting skin permeability of chemical mixtures from different vehicles. Toxicol. Sci. 2007;99:153–161. - PubMed
-
- Wolf S, Lohbrunner H, Busch T, Sterner-Kock A, Deja M, Sarrafzadeh A, Neumann U, Kaisers U. Small dose of exogenous surfactant combined with partial liquid ventilation in experimental acute lung injury: effects on gas exchange, haemodynamics, lung mechanics, and lung pathology. Br. J. Anaesth. 2001;87:593–601. - PubMed
-
- Fendler JH. Atomic and molecular clusters in membrane mimetic chemistry. Chem. Rev. 1987;87:877–899.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

