Depletion of regulatory T cells by anti-GITR mAb as a novel mechanism for cancer immunotherapy
- PMID: 20480365
- PMCID: PMC11030908
- DOI: 10.1007/s00262-010-0866-5
Depletion of regulatory T cells by anti-GITR mAb as a novel mechanism for cancer immunotherapy
Abstract
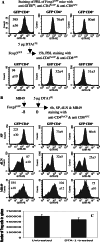
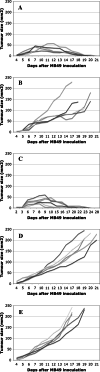
In vitro, engagement of GITR on Treg cells by the agonistic anti-GITR mAb, DTA-1, appears to abrogate their suppressive function. The consequence of in vivo engagement of GITR by DTA-1 is, however, less clear. In this study, we show that Treg cells isolated from DTA-1-treated mice were as potent as those from untreated mice in suppressing conventional CD4 T cells in vitro, indicating that in vivo GITR ligation does not disable Treg cells. Treatment of Foxp3/GFP knock-in mice with DTA-1 led to a selective reduction of circulating Treg cells, suggesting that DTA-1 is a depleting mAb which preferentially targets Treg cells. In tumour-bearing mice, DTA-1-mediated depletion of Treg cells was most marked in tumours but not in tumour-draining lymph node. These features were confirmed in an adoptive transfer model using tumour antigen-specific Treg cells. Interestingly, Treg cells detected in tumour tissues expressed much higher levels of GITR than those in tumour-draining lymph nodes, indicating that the efficiency of depletion might be correlated with the level of GITR expression. Finally, in vivo labelling of GITR in naive or tumour-bearing mice demonstrated that Treg cells constitutively expressed higher levels of GITR than conventional T cells, independent of location and activation state, consistent with the preferential in vivo depletion of Tregs by DTA-1. Thus, depletion of Treg cells represents a previously unrecognised in vivo activity of DTA-1 which has important implications for the application of anti-GITR antibodies in cancer immunotherapy.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

