Manganese catalysts for C-H activation: an experimental/theoretical study identifies the stereoelectronic factor that controls the switch between hydroxylation and desaturation pathways
- PMID: 20481432
- PMCID: PMC2903729
- DOI: 10.1021/ja908744w
Manganese catalysts for C-H activation: an experimental/theoretical study identifies the stereoelectronic factor that controls the switch between hydroxylation and desaturation pathways
Abstract
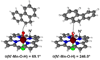
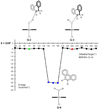
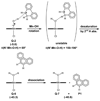
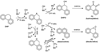
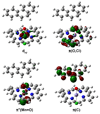
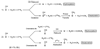
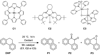
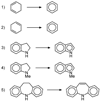
We describe competitive C-H bond activation chemistry of two types, desaturation and hydroxylation, using synthetic manganese catalysts with several substrates. 9,10-Dihydrophenanthrene (DHP) gives the highest desaturation activity, the final products being phenanthrene (P1) and phenanthrene 9,10-oxide (P3), the latter being thought to arise from epoxidation of some of the phenanthrene. The hydroxylase pathway also occurs as suggested by the presence of the dione product, phenanthrene-9,10-dione (P2), thought to arise from further oxidation of hydroxylation intermediate 9-hydroxy-9,10-dihydrophenanthrene. The experimental work together with the density functional theory (DFT) calculations shows that the postulated Mn oxo active species, [Mn(O)(tpp)(Cl)] (tpp = tetraphenylporphyrin), can promote the oxidation of dihydrophenanthrene by either desaturation or hydroxylation pathways. The calculations show that these two competing reactions have a common initial step, radical H abstraction from one of the DHP sp(3) C-H bonds. The resulting Mn hydroxo intermediate is capable of promoting not only OH rebound (hydroxylation) but also a second H abstraction adjacent to the first (desaturation). Like the active Mn(V)=O species, this Mn(IV)-OH species also has radical character on oxygen and can thus give H abstraction. Both steps have very low and therefore very similar energy barriers, leading to a product mixture. Since the radical character of the catalyst is located on the oxygen p orbital perpendicular to the Mn(IV)-OH plane, the orientation of the organic radical with respect to this plane determines which reaction, desaturation or hydroxylation, will occur. Stereoelectronic factors such as the rotational orientation of the OH group in the enzyme active site are thus likely to constitute the switch between hydroxylase and desaturase behavior.
Figures



Similar articles
-
Beyond ferryl-mediated hydroxylation: 40 years of the rebound mechanism and C-H activation.J Biol Inorg Chem. 2017 Apr;22(2-3):185-207. doi: 10.1007/s00775-016-1414-3. Epub 2016 Dec 1. J Biol Inorg Chem. 2017. PMID: 27909920 Free PMC article. Review.
-
Manganese-Oxygen Intermediates in O-O Bond Activation and Hydrogen-Atom Transfer Reactions.Acc Chem Res. 2017 Nov 21;50(11):2706-2717. doi: 10.1021/acs.accounts.7b00343. Epub 2017 Oct 24. Acc Chem Res. 2017. PMID: 29064667 Review.
-
Mechanistic Insights into the Selectivity of Norcarane Oxidation by Oxo-Manganese(V) Porphyrin Complexes.Chemphyschem. 2022 Mar 4;23(5):e202100810. doi: 10.1002/cphc.202100810. Epub 2022 Feb 1. Chemphyschem. 2022. PMID: 34981629
-
Understanding the oxidative relationships of the metal oxo, hydroxo, and hydroperoxide intermediates with manganese(IV) complexes having bridged cyclams: correlation of the physicochemical properties with reactivity.Acc Chem Res. 2013 Feb 19;46(2):483-92. doi: 10.1021/ar300208z. Epub 2012 Nov 29. Acc Chem Res. 2013. PMID: 23194251
-
Manganese Catalyzed C-H Halogenation.Acc Chem Res. 2015 Jun 16;48(6):1727-35. doi: 10.1021/acs.accounts.5b00062. Epub 2015 Jun 4. Acc Chem Res. 2015. PMID: 26042637
Cited by
-
Intramolecular gas-phase reactions of synthetic nonheme oxoiron(IV) ions: proximity and spin-state reactivity rules.Chemistry. 2012 Sep 10;18(37):11747-60. doi: 10.1002/chem.201200105. Epub 2012 Jul 26. Chemistry. 2012. PMID: 22837063 Free PMC article.
-
On how the binding cavity of AsqJ dioxygenase controls the desaturation reaction regioselectivity: a QM/MM study.J Biol Inorg Chem. 2018 Jul;23(5):795-808. doi: 10.1007/s00775-018-1575-3. Epub 2018 Jun 6. J Biol Inorg Chem. 2018. PMID: 29876666 Free PMC article.
-
Beyond ferryl-mediated hydroxylation: 40 years of the rebound mechanism and C-H activation.J Biol Inorg Chem. 2017 Apr;22(2-3):185-207. doi: 10.1007/s00775-016-1414-3. Epub 2016 Dec 1. J Biol Inorg Chem. 2017. PMID: 27909920 Free PMC article. Review.
-
Copper-catalysed dehydrogenation or lactonization of C(sp3)-H bonds.Nature. 2024 May;629(8011):363-369. doi: 10.1038/s41586-024-07341-z. Epub 2024 Mar 28. Nature. 2024. PMID: 38547926
-
Singlet versus Triplet Reactivity in an Mn(V)-Oxo Species: Testing Theoretical Predictions Against Experimental Evidence.J Am Chem Soc. 2016 Sep 28;138(38):12375-86. doi: 10.1021/jacs.6b05027. Epub 2016 Sep 14. J Am Chem Soc. 2016. PMID: 27545752 Free PMC article.
References
-
- Hill HAO, Sadler PJ, Thomson AJ, editors. Metal Sites in Proteins and Models: Iron Centres (Structure and Bonding, Vol. 88) Emeryville: Springer-Verlag Telos; 1997.
-
- Ozaki SI, Roach MP, Matsui T, Watanabe Y. Acc. Chem. Res. 2001;34:818–825. - PubMed
- Poulos TL, Li HY, Raman CS, Schuller DJ. Adv. Inorg. Chem. 2001;51:243–293.
- Groves JT. Proc. Natl. Acad. Sci. U. S. A. 2003;100:3569–3574. - PMC - PubMed
- Matsunaga I, Shir Y. Curr. Opin. Chem. Biol. 2004;8:127–132. - PubMed
- Meunier B, de Visser SP, Shaik S. Chem. Rev. 2004;104:3947–3980. - PubMed
- Ortiz de Montellano PR. Cytochrome P450: Structure, Mechanism, and Biochemistry. 3rd ed. New York: Kluwer Academic/Plenum Publishers; 2005.
- Denisov IG, Makris TM, Sligar SG, Schlichting I. Chem. Rev. 2005;105:2253–2278. - PubMed
- Solomon EI, Sundaram UM, Machonkin TE. Chem. Rev. 1996;96:2563–2605. - PubMed
- Que L, Dong YH. Acc. Chem. Res. 1996;29:190–196.
- Sazinsky MH, Lippard SJ. Acc. Chem. Res. 2006;39:558–566. - PubMed
- Shu LJ, Nesheim JC, Kauffmann K, Munck E, Lipscomb JD, Que L. Science. 1997;275:515–518. - PubMed
-
- Bloch K. Acc. Chem. Res. 1969;2:193–202.
- Fox BG, Lyle KS, Rogge CE. Acc. Chem. Res. 2004;37:421–429. - PubMed
- Behrouzian B, Buist PH. Curr. Opin. Chem. Biol. 2002;6:577–582. - PubMed
- Buist PH. Nat. Prod. Rep. 2004;21:249–262. - PubMed
- Abad JL, Camps F, Fabriàs G. Angew. Chem., Int. Ed. 2000;39:3279–3281. - PubMed
- Abad JL, Camps F, Fabriàs G. J. Am. Chem. Soc. 2007;129:15007–15012. - PubMed
- Shanklin J, Guy JE, Mishra G, Lindqvist Y. J. Biol. Chem. 2009;284:18559–18563. - PMC - PubMed
- Johansson AJ, Blomberg MRA, Siegbahn PEM. J. Phys. Chem. C. 2007;111:12397–12406.
-
- Groves JT, McClusky GA. J. Am. Chem. Soc. 1976;98:859–861.
- Groves JT. J. Chem. Educ. 1985;62:928–931.
-
- Buist PH, Marecak DM. J. Am. Chem. Soc. 1992;114:5073–5080.
- Akhtar M, Wright JN. Nat. Prod. Rep. 1991;8:527–551. - PubMed
- Cook GK, Mayer JM. J. Am. Chem. Soc. 1994;116:1855–1868.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

