Stereochemical and skeletal diversity arising from amino propargylic alcohols
- PMID: 20481457
- PMCID: PMC2883853
- DOI: 10.1021/ol100914b
Stereochemical and skeletal diversity arising from amino propargylic alcohols
Abstract
An efficient synthetic pathway to the possible stereoisomers of skeletally diverse heterocyclic small molecules is presented. The change in shape brought about by different intramolecular cyclizations of diastereoisomeric amino propargylic alcohols is quantified using principal moment-of-inertia (PMI) shape analysis.
Figures
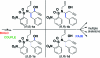


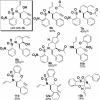
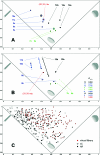
References
-
- Schreiber S. L. ChemBioChem 2009, 10, 26–29.
-
- Schreiber S. L. Nature 2009, 153–154. - PubMed
-
- Payne D. J.; Gwynn M. N.; Holmes D. L.; Pompliano D. L. Nat. Rev. Drug Discovery 2007, 6, 29–40. - PubMed
-
-
For recent examples, see:
- Kumagai N.; Muncipinto G.; Schreiber S. L. Angew. Chem. Int. Ed 2006, 45, 3635–3638. - PubMed
- Spiegel D. A.; Schroeder F. C.; Duvall J. R.; Schreiber S. L. J. Am. Chem. Soc. 2006, 128, 14766–14767. - PubMed
- Uchida T.; Rodriquez M.; Schreiber S. L. Org. Lett. 2009, 11, 1559–1562. - PMC - PubMed
-
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

