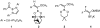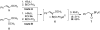Synthesis of an acyltrifluoroborate and its fusion with azides to form amides
- PMID: 20481486
- PMCID: PMC2896291
- DOI: 10.1021/jo1004058
Synthesis of an acyltrifluoroborate and its fusion with azides to form amides
Abstract
A uniquely stable acyl potassium trifluoroborate, potassium (2-phenylacetyl)trifluoroborate, has been synthesized and isolated. In the presence of an activating Lewis acid, this reagent reacts with azides to form amides in good yields.
Figures
References
-
- Hall DG. Boronic Acids: Preparation and Applications in Organic Synthesis and Medicine. Wiley-VCH; New York: 2005.
-
- Miyaura N. In: Metal-Catalyzed Cross-Coupling Reactions. Diederich F, de Meijere A, editors. Chapter 2 Wiley-VCH; New York: 2004.
-
- Miyaura N, Suzuki A. Chem Rev. 1995;95:2457–2483.
-
- McReynolds MD, Hanson PR. Chemtracts. 2001;14:796–801.
-
- Hayashi T, Yamasaki K. Chem Rev. 2003;103:2829–2844. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources