Immune privilege of corneal allografts
- PMID: 20482389
- PMCID: PMC3564633
- DOI: 10.3109/09273948.2010.486100
Immune privilege of corneal allografts
Abstract
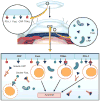
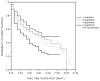
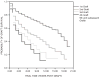
Corneal transplantation has been performed successfully for over 100 years. Normally, HLA typing and systemic immunosuppressive drugs are not utilized, yet 90% of corneal allografts survive. In rodents, corneal allografts representing maximal histoincompatibility enjoy >50% survival even without immunosuppressive drugs. By contrast, other categories of transplants are invariably rejected in such donor/host combinations. The acceptance of corneal allografts compared to other categories of allografts is called immune privilege. The cornea expresses factors that contribute to immune privilege by preventing the induction and expression of immune responses to histocompatibility antigens on the corneal allograft. Among these are soluble and cell membrane molecules that block immune effector elements and also apoptosis of T lymphocytes. However, some conditions rob the corneal allograft of its immune privilege and promote rejection, which remains the leading cause of corneal allograft failure. Recent studies have examined new strategies for restoring immune privilege to such high-risk hosts.
Conflict of interest statement
Figures

References
-
- America EBA. Statistical Report on Eye Banking Activity for 2008. www.restoresightorg/donation/statisticshtm2008.
-
- Zirm E. Eine erfolgreiche totale Keratoplastik. Albrecht von Graefes Arch Ophthalmol. 1906;64:580–593.
-
- Billingham RE, Boswell T. Studies on the problem of corneal homografts. Proc R Soc Lond B Biol Sci. 1953;141(904):392–406. - PubMed
-
- Niederkorn JY. Immunoregulation of intraocular tumours. Eye. 1997;11(Pt 2):249–254. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
