Emergence and migration of trunk neural crest cells in a snake, the California Kingsnake (Lampropeltis getula californiae)
- PMID: 20482793
- PMCID: PMC2886003
- DOI: 10.1186/1471-213X-10-52
Emergence and migration of trunk neural crest cells in a snake, the California Kingsnake (Lampropeltis getula californiae)
Abstract
Background: The neural crest is a group of multipotent cells that emerges after an epithelial-to-mesenchymal transition from the dorsal neural tube early during development. These cells then migrate throughout the embryo, giving rise to a wide variety derivatives including the peripheral nervous system, craniofacial skeleton, pigment cells, and endocrine organs. While much is known about neural crest cells in mammals, birds, amphibians and fish, relatively little is known about their development in non-avian reptiles like snakes and lizards.
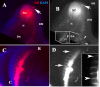
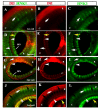
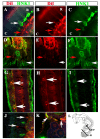
Results: In this study, we show for the first time ever trunk neural crest migration in a snake by labeling it with DiI and immunofluorescence. As in birds and mammals, we find that early migrating trunk neural crest cells use both a ventromedial pathway and an inter-somitic pathway in the snake. However, unlike birds and mammals, we also observed large numbers of late migrating neural crest cells utilizing the inter-somitic pathway in snake.
Conclusions: We found that while trunk neural crest migration in snakes is very similar to that of other amniotes, the inter-somitic pathway is used more extensively by late-migrating trunk neural crest cells in snake.
Figures





Similar articles
-
The development of the trunk neural crest in the turtle Trachemys scripta.Dev Dyn. 2020 Jan;249(1):125-140. doi: 10.1002/dvdy.119. Epub 2019 Oct 9. Dev Dyn. 2020. PMID: 31587387 Free PMC article.
-
Chicken trunk neural crest migration visualized with HNK1.Acta Histochem. 2015 Apr;117(3):255-66. doi: 10.1016/j.acthis.2015.03.002. Epub 2015 Mar 21. Acta Histochem. 2015. PMID: 25805416 Free PMC article.
-
Segmental organization of neural crest migration.Mech Dev. 2001 Jul;105(1-2):37-45. doi: 10.1016/s0925-4773(01)00395-1. Mech Dev. 2001. PMID: 11429280 Review.
-
Migrating neural crest cells in the trunk of the avian embryo are multipotent.Development. 1991 Aug;112(4):913-20. doi: 10.1242/dev.112.4.913. Development. 1991. PMID: 1935700
-
Inhibitory interactions in the patterning of trunk neural crest migration.Ann N Y Acad Sci. 1998 Oct 23;857:13-22. doi: 10.1111/j.1749-6632.1998.tb10103.x. Ann N Y Acad Sci. 1998. PMID: 10026081 Review.
Cited by
-
Gene duplication of endothelin 3 is closely correlated with the hyperpigmentation of the internal organs (Fibromelanosis) in silky chickens.Genetics. 2012 Feb;190(2):627-38. doi: 10.1534/genetics.111.136705. Epub 2011 Nov 30. Genetics. 2012. PMID: 22135351 Free PMC article.
-
A single locus regulates a female-limited color pattern polymorphism in a reptile.Sci Adv. 2022 Mar 11;8(10):eabm2387. doi: 10.1126/sciadv.abm2387. Epub 2022 Mar 9. Sci Adv. 2022. PMID: 35263124 Free PMC article.
-
Physiological electric fields induce directional migration of mammalian cranial neural crest cells.Dev Biol. 2021 Mar;471:97-105. doi: 10.1016/j.ydbio.2020.12.011. Epub 2020 Dec 24. Dev Biol. 2021. PMID: 33340512 Free PMC article.
-
Follow-the-leader cell migration requires biased cell-cell contact and local microenvironmental signals.Phys Biol. 2013 Jun;10(3):035003. doi: 10.1088/1478-3975/10/3/035003. Epub 2013 Jun 4. Phys Biol. 2013. PMID: 23735560 Free PMC article.
-
Effects of environmental factors and intraspecific niche overlap on the body and ecological characteristics of red-tongued pit vipers (Gloydius ussuriensis).Sci Rep. 2023 Dec 3;13(1):21310. doi: 10.1038/s41598-023-48707-z. Sci Rep. 2023. PMID: 38042889 Free PMC article.
References
-
- Hall BK. The Neural Crest and Neural Crest Cells in Vertebrate Development and Evolution. New York: Springer-Verlag; 2009.
-
- Baker CV. Neural Crest and Cranial Ectodermal Placodes. 4. New York: Springer, New York; 2005.
-
- Smith M, Hickman A, Amanzee D, Lumsden A, Thorogood P. Trunk neural crest origin of caudal fin mesenchyme in the zebrafish Danio rerio. Proc R Soc Lond B Biol Sci. 1994;256:137–145. doi: 10.1098/rspb.1994.0061. - DOI
-
- Serbedzija GN, Bronner-Fraser M, Fraser SE. A vital dye analysis of the timing and pathways of avian trunk neural crest cell migration. Development. 1989;106:809–16. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

