Differential splicing of the apoptosis-associated speck like protein containing a caspase recruitment domain (ASC) regulates inflammasomes
- PMID: 20482797
- PMCID: PMC2887861
- DOI: 10.1186/1476-9255-7-23
Differential splicing of the apoptosis-associated speck like protein containing a caspase recruitment domain (ASC) regulates inflammasomes
Abstract
Background: The apoptotic speck-like protein containing a caspase recruitment domain (ASC) is the essential adaptor protein for caspase 1 mediated interleukin (IL)-1beta and IL-18 processing in inflammasomes. It bridges activated Nod like receptors (NLRs), which are a family of cytosolic pattern recognition receptors of the innate immune system, with caspase 1, resulting in caspase 1 activation and subsequent processing of caspase 1 substrates. Hence, macrophages from ASC deficient mice are impaired in their ability to produce bioactive IL-1beta. Furthermore, we recently showed that ASC translocates from the nucleus to the cytosol in response to inflammatory stimulation in order to promote an inflammasome response, which triggers IL-1beta processing and secretion. However, the precise regulation of inflammasomes at the level of ASC is still not completely understood. In this study we identified and characterized three novel ASC isoforms for their ability to function as an inflammasome adaptor.
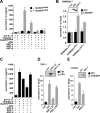
Methods: To establish the ability of ASC and ASC isoforms as functional inflammasome adaptors, IL-1beta processing and secretion was investigated by ELISA in inflammasome reconstitution assays, stable expression in THP-1 and J774A1 cells, and by restoring the lack of endogenous ASC in mouse RAW264.7 macrophages. In addition, the localization of ASC and ASC isoforms was determined by immunofluorescence staining.
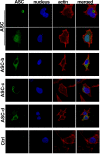
Results: The three novel ASC isoforms, ASC-b, ASC-c and ASC-d display unique and distinct capabilities to each other and to full length ASC in respect to their function as an inflammasome adaptor, with one of the isoforms even showing an inhibitory effect. Consistently, only the activating isoforms of ASC, ASC and ASC-b, co-localized with NLRP3 and caspase 1, while the inhibitory isoform ASC-c, co-localized only with caspase 1, but not with NLRP3. ASC-d did not co-localize with NLRP3 or with caspase 1 and consistently lacked the ability to function as an inflammasome adaptor and its precise function and relation to ASC will need further investigation.
Conclusions: Alternative splicing and potentially other editing mechanisms generate ASC isoforms with distinct abilities to function as inflammasome adaptor, which is potentially utilized to regulate inflammasomes during the inflammatory host response.
Figures




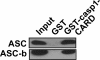
References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

